↧
Stability Data for ANDAs in the USA: a new Q&A Document of the FDA provides further Clarity « New Drug Approvals
↧
Stability Data for ANDAs in the USA: a new Q&A Document of the FDA provides further Clarity « New Drug Approvals
↧
↧
Summary of Translational Medicine – Cardiovascular Diseases – Part 1 « New Drug Approvals
↧
US Orphan Drug Market Outlook 2018 ……….download available « New Drug Approvals
↧
XenoPort begins phase II trial of XP-23829 in patients with psoriasis
XP 23829 from Xenoport is an interesting molecule and as on 27 July 2014, I did not find conclusive evidence
See some structures below
 Not sure about the structure of XP 23829
Not sure about the structure of XP 23829OR
OR
(N,N-dimethylcarbamoyl)methyl methyl(2E)but-2-ene-1,4-dioate.
OR
I AM NOT SURE ABOUT THIS ONE ALSO????????
As Football worldcup2014 goes on in Brazil

A thought for it is due............
............................................................
Best fit is probably is as shown below, and there are reasons
(N,N- Diethylcarbamoyl)methyl methyl (2E)but-2-ene-l,4-dioate ![]()

Introduction
(N,N-Diethylcarbamoyl)methyl methyl (2E)-but-2-ene-1,4-dioate
As per patent........http://www.google.com/patents/WO2014031844A1?cl=en![Links]()

C11 H17 N O5, mw 243.13
M.p.: 53-56 °C.
1 H NMR (CDCI3, 400 MHz): δ 6.99-6.90 (m, 2H), 4.83 (s, 2H), 3.80 (s, 3H), 3.39 (q, J = 1.1 Hz, 2H), 3.26 (q, J = 7.2 Hz, 2H), 1 .24 (t, J = 7.2 Hz, 3H), 1 .14 (t, J = 7.2 Hz, 3H). MS (ESI): m/z 244.13 (M+H)+.
Cas.......1208229-58-6
XP-23829 PROBABLE
For the treatment of moderate-to-severe chronic plaque-type psoriasis.
XP-H-093
US8148414![Links]() Basic patent
Basic patent
 Basic patent
Basic patentXenoport, Inc. Innovator
XenoPort has initiated a phase II trial of XP-23829, a proprietary investigational next-generation fumaric acid product candidate (ClinicalTrials.gov Identifier NCT02173301). The multicenter, randomized, double-blind, placebo-controlled study is designed to assess the efficacy and safety of XP-23829 as a potential treatment of patients with moderate to severe chronic plaque-type psoriasis. XenoPort expects to enroll approximately 200 subjects in this trial, which is being conducted in the U.S. The study will include a screening and washout phase of up to 4 weeks, a 12-week treatment phase and a 4-week post-treatment phase. Eligible study subjects will be randomized to placebo or one of three treatment arms of XP-23829: 400 or 800 mg once daily or 400 mg twice daily. The primary endpoint will examine the percent change in Psoriasis Area and Severity Index (PASI) score from baseline at the end of week 12. Secondary endpoints will include the proportion of subjects who achieve a reduction of 75% or greater from baseline in PASI (PASI75) score and subjects who achieve a Static Physicians Global Assessment score of "clear" or "almost clear." Topline results are expected in the third quarter of 2015 (XenoPort News Release).
XP23829 — A Prodrug of Monomethyl Fumarate
Our third product candidate, XP23829, is in Phase 1 clinical development. Provided we are able to demonstrate the safety and desired pharmacokinetic, or PK, profile of XP23829 in our Phase 1 trials, we believe that XP23829 could be a potential treatment of patients with RRMS, psoriasis and/or certain other disorders where the mechanism of action of XP23829 may be relevant. For example, we are exploring the potential of XP23829 to protect against neurodegeneration in experimental preclinical models of Parkinson’s disease through a grant from The Michael J. Fox Foundation. We hold a composition-of-matter patent and a formulation patent in the United States on XP23829 and hold patents or pending patent applications directed to the XP23829 methods of synthesis and use in the United States. We have also filed applications directed to the XP23829 composition of matter and methods of synthesis and use in other jurisdictions.
Prodrug Background
XP23829 is a fumaric acid ester compound and a patented prodrug of MMF. Fumaric acid ester compounds have shown immuno-modulatory and neuroprotective effects in cell-based systems and preclinical models of disease. A product containing a combination of fumaric acid ester compounds, known as Fumaderm, is approved in Germany for the treatment of psoriasis. Tecfidera (a formulation of DMF, also known as BG-12) from Biogen Idec Inc. is another fumaric acid ester prodrug that converts to MMF in the body. Phase 3 clinical trials of Tecfidera as a potential treatment for RRMS showed statistically significant benefits of Tecfidera versus placebo. Tecfidera is currently under U.S. regulatory review as a potential treatment for RRMS.
Our Prodrug
XP23829 is a novel prodrug of MMF that we believe may provide improved tolerability and efficacy compared to DMF. In preclinical studies that compared molar equivalent doses of XP23829 to DMF, XP23829 provided higher blood levels of the biologically active molecule MMF and a similar or greater degree of efficacy in MS and psoriasis animal models. Toxicology studies conducted in two species showed that XP23829 caused less stomach irritation when compared to DMF.
Phase 1 Clinical Trial in Healthy Volunteers
In October 2012, we reported favorable preliminary results from our first Phase 1 clinical trial in healthy adults designed to assess the pharmacokinetics, safety and tolerability of single doses of four different formulations of XP23829. The trial was a randomized, double-blind, two-period crossover, food effect comparison clinical trial of XP23829. Sixty subjects were assigned to five cohorts of 12, with each cohort receiving one of four different formulations of XP23829 or placebo. The trial demonstrated that administration of XP23829 resulted in the expected levels of MMF in the blood. As anticipated, the four formulations produced
.gif)
April 4, 2012
http://investor.xenoport.com/releasedetail.cfm?ReleaseID=708145![Links]()
XenoPort Awarded U.S. Patent Directed to Composition and Formulations of XP23829, a Novel Fumarate Analog for the Potential Treatment of Relapsing-Remitting Multiple Sclerosis and Psoriasis
SANTA CLARA, Calif.--(BUSINESS WIRE)--Apr. 4, 2012-- XenoPort, Inc. (Nasdaq: XNPT) announced today that it was awarded U.S. Patent 8,148,414 for "Prodrugs of Methyl Hydrogen Fumarate, Pharmaceutical Compositions Thereof, and Methods of Use." The term of the patent extends until 2029, subject to potential Hatch-Waxman patent term extensions.

XenoPort Awarded U.S. Patent Directed to Composition and Formulations of XP23829, a Novel Fumarate Analog for the Potential Treatment of Relapsing-Remitting Multiple Sclerosis and Psoriasis
SANTA CLARA, Calif.--(BUSINESS WIRE)--Apr. 4, 2012-- XenoPort, Inc. (Nasdaq: XNPT) announced today that it was awarded U.S. Patent 8,148,414 for "Prodrugs of Methyl Hydrogen Fumarate, Pharmaceutical Compositions Thereof, and Methods of Use." The term of the patent extends until 2029, subject to potential Hatch-Waxman patent term extensions.
The patent is directed to the XP23829 compound, analogs thereof and formulations thereof. A related U.S. patent application directed to therapeutic uses of XP23829 is now pending.
XP23829 is a prodrug of methyl hydrogen fumarate, also known as monomethyl fumarate (MMF). In cell- and animal-based models, MMF has been shown to exhibit immuno-modulatory properties and inhibit damage from oxidative stress.
In XenoPort's preclinical animal studies that compared molar equivalent doses of XP23829 to dimethyl fumarate (DMF), another prodrug of MMF, XP23829 demonstrated a greater degree of efficacy in animal models of both multiple sclerosis (MS) and psoriasis. Toxicology studies conducted in two species showed that XP23829 caused less stomach irritation compared to DMF.
XenoPort intends to file an Investigational New Drug Application (IND) for XP23829 for the treatment of relapsing remitting MS with the U.S. Food and Drug Administration (FDA) in the second quarter of 2012 and expects to initiate human clinical trials later this year.
XenoPort owns all rights to XP23829.
About XenoPort
XenoPort is a biopharmaceutical company focused on developing and commercializing a portfolio of internally discovered product candidates for the potential treatment of neurological disorders. Horizant® (gabapentin enacarbil) Extended-Release Tablets is XenoPort's first FDA-approved product. GlaxoSmithKline holds commercialization rights and certain development rights for Horizant in the United States. Regnite® (gabapentin enacarbil) is approved for the treatment of moderate-to-severe primary restless legs syndrome in Japan. Astellas Pharma Inc. holds all development and commercialization rights for Regnite in Japan and five Asian countries. XenoPort holds all other world-wide rights and has co-promotion and certain development rights to gabapentin enacarbil in the United States. XenoPort's pipeline of product candidates includes potential treatments for patients with postherpetic neuralgia, spasticity and Parkinson's disease.
More info about this drug
SEE a patent
WO 2010022177
..............................................
WO 2013181451
Scheme 5:
ONE OUT OF THESE
Example 6: (/V,/V-Diethylcarbamoyl)methyl methyl (2£)but-2-ene-1 ,4-dioate![]()

[0138] Following general procedure A, methyl hydrogen fumarate (MHF) (0.39 g, 3.00 mmol) dissolved in NMP was reacted at about 55 °C with 2-chloro-/V,/V-diethylacetamide (0.44 g, 3.00 mmol) in the presence of CsHC03 (0.69 g, 3.60 mmol) to afford 0.37 g (51 % yield) of the title compound after purification by silica gel column chromatography (Biotage) using a mixture of ethyl acetate (EtOAc) and hexanes (1 :1 ) as eluent. M.p.: 53-56 °C. 1 H NMR (CDCI3, 400 MHz): δ 6.99-6.90 (m, 2H), 4.83 (s, 2H), 3.80 (s, 3H), 3.39 (q, J = 1.1 Hz, 2H), 3.26 (q, J = 7.2 Hz, 2H), 1 .24 (t, J = 7.2 Hz, 3H), 1 .14 (t, J = 7.2 Hz, 3H). MS (ESI): m/z 244.13 (M+H)+.
Example 7: Methyl 2-morpholin-4-yl-2-oxoethyl (2 £)but-2-ene-1 ,4-dioate
[0139] Following general procedure A, methyl hydrogen fumarate (MHF) (0.50 g, 3.84 mmol) dissolved in NMP was reacted at about 55 °C with 4-(chloroacetyl) morpholine (0.75 g, 4.61 mmol) in the presence of CsHC03 (0.89 g, 4.61 mmol) to afford 0.34 g (35% yield) of the title compound as a white solid after purification by mass-guided preparative HPLC and lyophilization. M.p.: 124 to 126°C; 1 H NMR (CDCI3, 400 MHz): δ 6.97-6.91 (m, 2H), 4.84 (s, 2H), 3.82 (s, 3H), 3.72-3.70 (m, 4H), 3.64-3.62 (m, 2H), 3.46-3.41 (m, 2H). MS (ESI): m/z 258.04 (M+H)+. Example 8: A/,A/-Dimethylcarbamoyl)methyl methyl (2E)but-2-ene-1 ,4-dioate
[0140] Following general procedure A, methyl hydrogen fumarate (MHF) (0.50 g, 3.84 mmol) dissolved in NMP was reacted at about 55 °C with /V,/V-dimethyl chloroacetamide (0.56 g, 4.61 mmol) in the presence of CsHC03 (0.89 g, 4.61 mmol). The crude material was precipitated out from a mixture of ethyl acetate (EtOAc) and hexanes (Hxn) (1 :1 ) to provide a white solid. This solid was further dissolved in dichloromethane (DCM) and the organic layer washed with water. After removal of the solvents 0.55 g (67% yield) of the title compound was obtained as a white solid. 1 H NMR (CDCI3, 400 MHz): δ 6.98- 6.90 (m, 2H), 4.84 (s, 2H), 3.80 (s, 3H), 2.99-2.97 (2s, 6H). MS (ESI): m/z 216 (M+H)+.
Example 9: Methyl (2-morpholino-4-ylethyl) fumarate
[0141] Following general Procedure A, methyl hydrogen fumarate (MHF) dissolved in NMP is reacted at about 55 °C with 4-(chloroethyl) morpholine (0.75 g, 4.61 mmol) in the presence of CsHC03 to afford the title compound after purification by mass-guided preparative HPLC and lyophilization. Example 10: Methyl (3-mor holino-4-ylpropyl) fumarate
[0142] Following the procedure of Methyl (2-morpholino-4-ylethyl) fumarate, and replacing 4-(chloroethyl) morpholine with 4-(chloropropyl) morpholine provides the title compound.
Example 11 : Methyl (4-morpholino-4-ylbutyl) fumarate
[0143] Following the procedure of Methyl (2-morpholino-4-ylethyl) fumarate, and replacing 4-(chloroethyl) morpholine with 4-(chlorobutyl) morpholine provides the title compound. Example 12: Methyl 5-morpholino-4-ylpentyl) fumarate
[0144] Following the procedure of Methyl (2-morpholino-4-ylethyl) fumarate, and replacing 4-(chloroethyl) morpholine with 4-(chloropentyl) morpholine provides the title compound. Example 13: (A/-cyclopropyl-W-ethylcarbamoyl)methyl methyl 2(E)but-2-ene-1 ,4-dioate
[0145] Following the general procedure A, methyl hydrogen fumarate (MHF) (38.7 g, 0.297 mol) suspended in toluene (100 mL) was reacted at about 80 °C with 2-chloro-/V-cyclopropyl- N-ethylacetamide (48 g, 0.297 mol) in the presence of W,/V-diisopropylethylamine (DIEA; 42.3 g, 57 mL, 0.327 mol) to afford 50 g (63.3%) of the title compound after recrystallization using methyl ferf-butyl ether. The crystalline compound had a melting point of 92.1 °C. 1 H NMR (CDCI3, 400 MHz): δ 7.01 -6.92 (m, 2H), 4.99 (s, 2H), 3.81 (s, 3H), 3.44 (q, J = 7.2 Hz, 2H), 2.69-2.66 (m, 1 H), 1 .14 (t, J = 7.2 Hz, 3H), 0.94-0.91 (m, 2H), 0.83-0.81 (m, 2H). MS (ESI): m/z 256.2 (M+H)+.
Example 14: (/V-cyclopropyl-/V-methylcarbamoyl)methyl methyl 2(E)but-2-ene-1 , 4- dioate
[0146] Following general procedure A, methyl hydrogen fumarate (MHF) (38.7 g, 0.40 mol) suspended in toluene (100 mL) was reacted at about 80 °C with 2-chloro-/V-cyclopropyl-/V- methylacetamide (60 g, 0.40 mol) in the presence of Ν,Ν-diisopropylethylamine (DIEA; 57.8 g, 78 mL, 0.44 mol) to afford 50 g (50.86%) of the title compound after recrystallization using methyl fe/t-butyl ether. The crystalline compound had a melting point of 93.6 °C. 1 H NMR (CDCI3, 400 MHz): δ 7.01 -6.91 (m, 2H), 5.01 (s, 2H), 3.82 (s, 3H), 2.94 (s, 3H), 2.73-2.68 (m, 1 H), 0.94-0.86 (m, 2H), 0.83-0.78 (m, 2H). MS (ESI): m/z 242.2 (M+H)+.
Example 15: Methyl 2-oxo-2-pyrrolidinylethyl 2(E)but-2-ene-1 ,4-dioate
[0147] Following general procedure A, methyl hydrogen fumarate (MHF) (20.78 g, 0.159 mol) suspended in toluene (60 mL) was reacted at about 80 °C with 2-chloro-1 -pyrrolidin-1 -yl- ethanone (23.5 g, 0.159 mol) in the presence of N,N-diisopropylethylamine (DIEA; 22.69 g, 31 .5 mL, 0.175 mol) to afford 24 g (62.3%) of the title compound after recrystallization using methyl fe/t-butyl ether. The crystalline compound had a melting point of 102.1 °C. 1 H NMR (CDCI3, 400 MHz): δ 7.00-6.92 (m, 2H), 4.75 (s, 2H), 3.81 (s, 3H), 3.53-3.49 (t, J = 6.8 Hz, 2H), 3.42-3.39 (t, J = 6.8 Hz, 2H), 2.20-1 .97 (m, 2H), 1 .91 -1 .82 (m, 2H). MS (ESI): m/z 242 (M+H)+.
....................................
Patent
Example 1(N,N-Diethylcarbamoyl)methyl methyl(2E)but-2-ene-1,4-dioate (1)............. best fit![]()

Following general procedure A, methyl hydrogen fumarate (MHF) (0.39 g, 3.00 mmol) dissolved in NMP was reacted at ca. 55° C. with 2-chloro-N,N-diethylacetamide (0.44 g, 3.00 mmol) in the presence of CsHCO3 (0.69 g, 3.60 mmol) to afford 0.37 g (51% yield) of the title compound (1) after purification by silica gel column chromatography (Biotage) using a mixture of ethyl acetate (EtOAc) and hexanes (1:1) as eluent. M.p.: 53-56° C. 1H NMR (CDCl3, 400 MHz): δ 6.99-6.90 (m, 2H), 4.83 (s, 2H), 3.80 (s, 3H), 3.39 (q, J=7.2 Hz, 2H), 3.26 (q, J=7.2 Hz, 2H), 1.24 (t, J=7.2 Hz, 3H), 1.14 (t, J=7.2 Hz, 3H). MS (ESI): m/z 244.13 (M+H)+.
Example 162-(4-Acetylpiperazinyl)-2oxoethyl methyl(2E)but-2ene-1,4-dioate (16)
Methyl 2-oxo-2-piperazinylethyl(2E)but-2-ene-1,4-dioate hydrochloride (14) (0.20 g, 0.68 mmol) was reacted with acetyl chloride (AcCl) (0.60 mL, 0.66 g, 0.84 mmol) and diisopropylethylamine (0.70 mL, 0.52 g, 4.0 mmol) in dichloromethane (DCM). Following aqueous work-up, the crude product was purified by silica gel flash chromatography to afford 0.12 g (54% yield) of the title compound (16) as a white solid. 1H NMR (CDCl3, 400 MHz): δ 6.98-6.93 (m, 2H), 4.86 (s, 2H), 3.83 (s, 3H), 3.66 3.63 (m, 4H), 3.50-3.40 (m, 4H), 2.14 (s, 3H). MS (ESI): m/z 299.12 (M+H)+.
Example 9N,N-Dimethylcarbamoyl)methyl methyl(2E)but-2-ene-1,4-dioate (9)
Following general procedure A, methyl hydrogen fumarate (MHF) (0.50 g, 3.84 mmol) dissolved in NMP was reacted at ca. 55° C. with N,N-dimethyl chloroacetamide (0.56 g, 4.61 mmol) in the presence of CsHCO3 (0.89 g, 4.61 mmol). The crude material was precipitated out from a mixture of ethyl acetate (EtOAc) and hexanes (Hxn) (1:1) to provide a white solid. This solid was further dissolved in dichloromethane (DCM) and the organic layer washed with water. After removal of the solvents 0.55 g (67% yield) of the title compound (9) was obtained as a white solid. 1H NMR (CDCl3, 400 MHz): δ 6.98-6.90 (m, 2H), 4.84 (s, 2H), 3.80 (s, 3H), 2.99-2.97 (2s, 6H). MS (ESI): m/z 216 (M+H)+.
.............................

Compound (1).
Table 1 : Flushing Incidence as a Function of MMF Cmax
*Formulation 2 is the dosage form described in Example 10; Formulation 3 is the dosage form described in Example 3 ; Formulation 4 is the dosage form described in Example 5 ;
** maximum average Concentration; ***average Cmax; Poster (see above); Compound (1) referred to in the above table is an MMF prodrug of Formula (II); (N,N- Diethylcarbamoyl)methyl methyl (2£)but-2-ene-l,4-dioate having the following chemical structure:
Compound (1).![]()

The maximum slope values ( dose and ng) for different dosage treatments are given in Table 2. The Figures 15-16 show plots of maximum MMF slope vs flushing incidence. The curves in the figures were fitted using a Hill Emax model. Table 2
Compound, Flushing
Table 3: Composition of Enteric Coated Sustained Release Tablet (15% HPMC in Core)
Quantity Quantity
Component Manufacturer Role
(mg tablet) (%w/w)
Vertellus (Greensboro,
Triethyl Citrate Plasticizer 1.25 0.42
NC)
Emerson Resources Anti- tacking
PlasAC YL™ T20 2.41 0.80
(Norristown, PA) agent
Total Enteric
27.87 9.30 Coating
Total Tablet 334.69 111.68
[00191] The tablets were made according to the following steps. The core tablets were prepared using a wet granulation process. The granulation was performed in two batches at 456 g per batch. Compound (1) and hydroxypropyl cellulose were passed through a conical mill with a 610 micron round holed screen. Compound (1) and hydroxypropyl cellulose were then combined in a Key KG- 5 granulator bowl and mixed with water addition for approximately 7 minutes. The wet granules were dried in a Glatt GPCG-1 fluid bed dryer at 40 °C. The two portions of dried granules were sized by passing through a conical mill with an approximately 1300 micron grater type screen. The milled granules were blended with the hypromellose 2208, silicon dioxide, and lactose monohydrate for 10 minutes in an 8 quart (7.6 1) V-blender. This blend was passed through an 850 micron mesh screen. The magnesium stearate was passed through a 600 micron mesh screen and blended with the additional core materials in the V-blender for 5 minutes. Core tablets (299.69 mg) were compressed using a GlobePharma Minipress II rotary tablet press with 8.6 mm round concave tooling. The core tablets had a final mean hardness of approximately 12 kp. For the coating, an aqueous suspension was prepared by mixing with an impeller 63.8 g Opadry 03019184 with 770.7 g of purified water. The water contained in the suspension is removed during the film coating process and therefore not included in the final formulation in Table 3. The tablets were coated with the aqueous suspension in an O' Hara Technologies Labcoat M coater with a 12" (30.5 cm) diameter perforated pan until the desired weight gain of barrier coat was achieved. The coating process occurred at an inlet temperature of approximately 52 °C and an outlet temperature of 36 °C. After coating, the tablets were dried for 2 hours at 40 °C. An aqueous suspension was prepared by mixing with an impeller 405.1 g methacrylic acid copolymer dispersion, 6.3 g triethyl citrate, 60.6 g PlasACRYL™ T20 with 228.1 g water. The water contained in the methacrylic acid copolymer dispersion and the
PlasACRYL™ T20 is removed during the film coating process and therefore not included in the final formulation in Table 3. The tablets were coated with the aqueous suspension in the O' Hara Technologies Labcoat M coater until the desired weight gain of enteric film was achieved. The coating process occurred at an inlet temperature of approximately 40 °C and an outlet temperature of 30 °C. After coating, the tablets were dried for 2 hours at 40 °C.
Example 2
In Vitro Dissolution Profile of Example 1 Dosage Form
[00192] A two-stage dissolution method was used to determine the in vitro dissolution profile of dosage forms prepared according to Example 1. The 2-stage dissolution test was used to better approximate the pH conditions experienced by a dosage form after swallowing by a patient, i.e., low pH of the stomach followed by near neutral pH of the intestines. The dosage forms were first placed into a dissolution vessel (USP, Type I, basket) containing 750 mL of 0.1 N hydrochloric acid (pH 1.2). After 2 hours, 250 mL of 200 mM tribasic sodium phosphate was added to the vessel resulting in a pH adjustment from 1.2 to 6.8. The dissolution medium was kept at 37 °C and was agitated at 100 rpm.
[00193] For the Example 1 dosage forms, samples of the dissolution medium were withdrawn after 1 and 2 hours in the low pH stage, and at 0.5, 2, 4, 7, 10, and 14 hours following buffer addition. The released amount of the MMF prodrug in the samples was determined by reverse phase HPLC using a C18 column and a 7 minute gradient method according to Table 4 where Mobile Phase A is water/0.1 ]¾Ρθ4 and Mobile Phase B is water/acetonitrile/H3PC>4 (10/90/0.1 by volume) with UV detection at 210 nm.
Table 4: HPLC Gradient Conditions
[00194] As shown in FIG. 1, for dosage forms prepared according to Example 1, drug release is delayed for approximately 2 hours, followed by sustained release reaching >90 at 12 hours.
Example 3
Preparation of Delayed Sustained Release Dosage Form (Enteric Coated, 15% HPMC in Core, without Barrier Layer) [00195] Delayed sustained release tablets containing compound (1) were made having the ingredients shown in Table 5:
Table 5: Composition of Enteric Coated Sustained Release Tablet (15% HPMC in Core, without Barrier Layer)
[00196] The tablets were made according to the following steps. The core tablets were prepared using a wet granulation process. The granulation was performed in two batches at 463.9 g per batch. Compound (1) and hydroxypropyl cellulose were passed through a conical mill with a 610 micron round holed screen. Compound (1) and hydroxypropyl cellulose were then combined in a Key KG- 5 granulator bowl and mixed with water addition for approximately 10 minutes. The wet granules were dried in a Glatt GPCG-1 fluid bed dryer at 40 °C. The two portions of dried granules were blended with silicon dioxide and sized by passing through a conical mill with an approximately 1300 micron grater type screen. The milled granules were blended with the hypromellose 2208 and lactose monohydrate for 10 minutes in an 8 quart (7.6 1) V-blender. This blend was passed through an 850 micron mesh screen. The magnesium stearate was passed through a 600 micron mesh screen and blended with the additional core materials in the V-blender for 5 minutes. Core tablets (299.68 mg) were compressed using a GlobePharma Minipress II rotary tablet press with 11/32" round concave tooling. The core tablets had a final mean hardness of approximately 11 kp. For the coating, an aqueous suspension was prepared by mixing with an impeller 578.7 g methacrylic acid copolymer dispersion, 9.0 g triethyl citrate, 86.5 g PlasACRYL™ T20 with 325.8 g water. The water contained in the methacrylic acid copolymer dispersion and the
PlasACRYL™ T20 is removed during the film coating process and therefore not included in the final formulation in Table 4. The tablets were coated with the aqueous suspension in the O' Hara Technologies Labcoat M coater until the desired weight gain of enteric film was achieved. The coating process occurred at an inlet temperature of approximately 41 °C and an outlet temperature of 31 °C. After coating, the tablets were dried for 2 hours at 40 °C.
..............................

WO 2014071371
(N,N-Diethylcarbamoyl)methyl methyl (2E)but-2-ene-1 ,4-dioate has the following chemical structure:
This compound was synthesized in Example 1 of Gangakhedkar et al., U.S. Patent No. 8,148,414. The compound is a prodrug of methyl hydrogen fumarate (MHF) and has a disclosed melting point of between 53 °C and 56 °C.
Cocrystals are crystals that contain two or more non-identical molecules that form a crystalline structure. The intermolecular interactions between the non-identical molecules in the resulting crystal structures can result in physical and chemical properties that differ from the properties of the individual components. Such properties can include, for example, melting point, solubility, chemical stability, mechanical properties and others. Examples of cocrystals may be found in the Cambridge Structural Database and in Etter, et al.,
"The use of cocrystallization as a method of studying hydrogen bond preferences of 2-aminopyridine" J. Chem. Soc, Chem. Commun. (1990), 589-591 ; Etter, et al., "Graph-set analysis of hydrogen-bond patterns in organic crystals" Acta Crystallogr., Sect. B, Struct. Sci. (1990), B46: 256-262; and Etter, et al., "Hydrogen bond directed cocrystallization and molecular recognition properties of diarylureas" J. Am. Chem. Soc. (1990), 1 12: 8415-8426. Additional information relating to cocrystals can be found in: Carl Henrik Gorbotz and Hans-Petter Hersleth,
"On the inclusion of solvent molecules in the crystal structures of organic compounds"; Acta Cryst. (2000), B56: 625-534; and Senthil Kumar, et al., "Molecular Complexes of Some Mono- and Dicarboxylic Acids with trans-1 ,4,-Dithiane-1 ,4-dioxide" American Chemical Society, Crystal Growth & Design (2002) , 2(4) : 313-318.
(N,N-Diethylcarbamoyl)methyl methyl (2E)but-2-ene-1 ,4-dioate is a prodrug of methyl hydrogen fumarate. Once administered, the compound is metabolized in vivo into an active metabolite, namely, methyl hydrogen fumarate (MHF) which is also referred to herein as monomethyl fumarate (MMF). The in vivo metabolism of (N,N-Diethylcarbamoyl)methyl
(N,N-Diethylcarbamoyl)methyl methyl Methyl hydrogen fumarate N ^ diethyl glycolamide
(2E)but-2-ene-1 ,4-dioate
Table 1
As can be seen from the data in Table 1 , the six cocrystals disclosed herein each exhibit a higher melting point than crystalline (N,N-Diethylcarbamoyl)methyl methyl (2E)but-2-ene-1 ,4- dioate.

..................................
Steady state pharmacokinetics of formulations of XP23829, a novel prodrug of monomethyl fumarate (MMF), in healthy subjects
66th Annu Meet Am Acad Neurol (AAN) (April 26-May 3, Philadelphia) 2014, Abst P1.188
66th Annu Meet Am Acad Neurol (AAN) (April 26-May 3, Philadelphia) 2014, Abst P1.188
........................................
Lymphocyte and eosinophil responses in healthy subjects dosed with Tecfidera and XP23829, a novel fumaric acid ester (FAE)
66th Annu Meet Am Acad Neurol (AAN) (April 26-May 3, Philadelphia) 2014, Abst P1.201
66th Annu Meet Am Acad Neurol (AAN) (April 26-May 3, Philadelphia) 2014, Abst P1.201
.............................
A comparison of XP23829 with DMF, the active ingredient of BG-12
4th Cooperative Meet Consorti Mult Scler Cent (CMSC) Am Comm Treat Res Mult Scler (ACTRIMS) (May 30-June 2, San Diego) 2012, Abst SC03
4th Cooperative Meet Consorti Mult Scler Cent (CMSC) Am Comm Treat Res Mult Scler (ACTRIMS) (May 30-June 2, San Diego) 2012, Abst SC03
................................
Favorable metabolism and pharmacokinetics of formulations of XP23829, a novel fumaric acid ester, in healthy subjects
65th Annu Meet Am Acad Neurol (AAN) (March 16-23, San Diego) 2013, Abst P05.189
65th Annu Meet Am Acad Neurol (AAN) (March 16-23, San Diego) 2013, Abst P05.189
.........................................
Comparison of the efficacy and tolerability of a novel methyl hydrogenfumarate prodrug with dimethyl fumarate in rodent EAE and GI irritation models
Neurology 2011, 76(9): Abst P05.040
Neurology 2011, 76(9): Abst P05.040
| WO2013119791A1 * | Feb 7, 2013 | Aug 15, 2013 | Xenoport, Inc. | Morpholinoalkyl fumarate compounds, pharmaceutical compositions, and methods of use |
| US20120034303 * | Jan 8, 2010 | Feb 9, 2012 | Forward Pharma A/S | Pharmaceutical formulation comprising one or more fumaric acid esters in an erosion matrix |
| US20120095003 * | Oct 14, 2011 | Apr 19, 2012 | Xenoport, Inc. | Methods of using prodrugs of methyl hydrogen fumarate and pharmaceutical compositions thereof |
| US20120157523 * | Oct 14, 2011 | Jun 21, 2012 | Xenoport, Inc. | Prodrugs of methyl hydrogen fumarate, pharmaceutical compositions thereof, and methods of use |
| K Gogas ET AL: "Comparison of the efficacy and tolerability of a novel methylhydrogenfumarate prodrug with dimethylfumarate in rodent experimental autoimmune encephalomyelitis and GI irritation models", 26th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) & 15th Annual Conference of Rehabilitation in MS (RIMS), 15 October 2010 (2010-10-15), XP055076728, Retrieved from the Internet: URL:http://registration.akm.ch/einsicht.php?XNABSTRACT_ID=115706&XNSPRACHE_ID=2&XNKONGRESS_ID=126&XNMASKEN_ID=900 [retrieved on 2013-08-27] |
| WO2013119791A1 * | Feb 7, 2013 | Aug 15, 2013 | Xenoport, Inc. | Morpholinoalkyl fumarate compounds, pharmaceutical compositions, and methods of use |
| US20100048651 * | Aug 19, 2009 | Feb 25, 2010 | Xenoport, Inc. | Prodrugs of methyl hydrogen fumarate, pharmaceutical compositions thereof, and methods of use |
| US8669281 | 20 Sep 2013 | 11 Mar 2014 | Alkermes Pharma Ireland Limited | Prodrugs of fumarates and their use in treating various diseases |
| WO2014031894A1 | 22 Aug 2013 | 27 Feb 2014 | Xenoport, Inc. | Oral dosage forms of methyl hydrogen fumarate and prodrugs thereof |
| WO2014071371A1 | 5 Nov 2013 | 8 May 2014 | Xenoport, Inc. | Cocrystals of (n,n-diethylcarbamoyl)methyl methyl (2e)but-2-ene-1,4-dioate |
↧
↧
BI launches COPD drug Striverdi, olodaterol in UK and Ireland

Olodaterol
BI-1744
BI-1744-CL (hydrochloride) marketed as drug
BI-1744-CL (hydrochloride) marketed as drug
Olodaterol (trade name Striverdi) is a long acting beta-adrenoceptor agonist used as an inhalation for treating patients with chronic obstructive pulmonary disease (COPD), manufactured by Boehringer-Ingelheim.[1]
Olodaterol is a potent agonist of the human β2-adrenoceptor with a high β1/β2 selectivity. Its crystalline hydrochloride salt is suitable for inhalation and is currently undergoing clinical trials in man for the treatment of asthma. Olodaterol has a duration of action that exceeds 24 hours in two preclinical animal models of bronchoprotection and it has a better safety margin compared with formoterol.
Bi 1744 cl
Bi-1744-cl
Olodaterol hydrochloride
Olodaterol hydrochloride [usan]
UNII-65R445W3V9
Bi-1744-cl
Olodaterol hydrochloride
Olodaterol hydrochloride [usan]
UNII-65R445W3V9
CAS 869477-96-3
R ENANTIOMER
2H-1,4-Benzoxazin-3(4H)-one, 6-hydroxy-8-((1R)-1-hydroxy-2-((2-(4-methoxyphenyl)- 1,1-dimethylethyl)amino)ethyl)-, hydrochloride (1:1)
2H-1,4-benzoxazin-3(4H)-one, 6-hydroxy-8-((1R)-1-hydroxy-2-((2-(4-methoxyphenyl)- 1,1-dimethylethyl)amino)ethyl)-, hydrochloride (1:1)
6-Hydroxy-8-((1R)-1-hydroxy-2-((2-(4-methoxyphenyl)-1,1-dimethylethyl)amino)ethyl)- 2H-1,4-benzoxazin-3(4H)-one hydrochloride
Boehringer Ingelheim has launched a new chronic obstructive pulmonary disease drug, Striverdi in the UK and Ireland.
Striverdi (olodaterol) is the second molecule to be licenced for delivery via the company’s Respimat Soft Mist inhaler, following the COPD blockbuster Spiriva (tiotropium). The drug was approved in Europe in November based on results from a Phase III programme that included more than 3,000 patients with moderate to very severe disease.http://www.pharmatimes.com/Article/14-07-01/BI_launches_COPD_drug_Striverdi_in_UK_and_Ireland.aspx
Striverdi (olodaterol) is the second molecule to be licenced for delivery via the company’s Respimat Soft Mist inhaler, following the COPD blockbuster Spiriva (tiotropium). The drug was approved in Europe in November based on results from a Phase III programme that included more than 3,000 patients with moderate to very severe disease.http://www.pharmatimes.com/Article/14-07-01/BI_launches_COPD_drug_Striverdi_in_UK_and_Ireland.aspx
Olodaterol hydrochloride is a drug candidate originated by Boehringer Ingelheim. The product, delivered once-daily by the Respimat Soft Mist Inhaler, was first launched in Denmark and the Netherlands in March 2014 for the use as maintenance treatment of chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema. In 2013, approval was obtained in Russia and Canada for the same indication, and in the U.S, the product was recommended for approval. Phase III clinical trials for the treatment of COPD are ongoing in Japan.
| Systematic (IUPAC) name | |
|---|---|
| 6-hydroxy-8-{(1R)-1-hydroxy-2-{[1-(4-methoxyphenyl)-2-methylpropan-2-yl]amino}ethyl}-4H-1,4-benzoxazin-3-one | |
| Clinical data | |
| Trade names | Striverdi |
| AHFS/Drugs.com | UK Drug Information |
| Pregnancy cat. | No experience |
| Legal status | POM (UK) |
| Routes | Inhalation |
| Identifiers | |
| CAS number | 868049-49-4; 869477-96-3 (hydrochloride) |
| ATC code | R03AC19 |
| PubChem | CID 11504295 |
| ChemSpider | 9679097 |
| UNII | VD2YSN1AFD |
| ChEMBL | CHEMBL605846 |
| Synonyms | BI 1744 CL |
| Chemical data | |
| Formula | C21H26N2O5 free formC21 H26 N2 O5 . Cl H; of hcl salt |
| Mol. mass | 386.44 g/mol free form; 422.902 as hyd salt |
Medical uses
Olodaterol is a once-daily maintenance bronchodilator treatment of airflow obstruction in patients with COPD including chronic bronchitis and/or emphysema, and is administered in an inhaler called Respimat Soft Mist Inhaler.[2][3][4][5][6][7]
As of December 2013, olodaterol is not approved for the treatment of asthma. Olodaterol monotherapy was previously evaluated in four Phase 2 studies in asthma patients. However, currently there are no Phase 3 studies planned for olodaterol monotherapy in patients with asthma.
In late January 2013, Olodaterol CAS# 868049-49-4 was the focus of an FDA committee reviewing data for the drug’s approval as a once-daily maintenance bronchodilator to treat chronic obstructive pulmonary disease (COPD), as well as chronic bronchitis and emphysema. The FDA Pulmonary-Allergy Drugs Advisory Committee recommended that the clinical data from the Boehringer Ingelheim Phase III studies be included in their NDA.
Also known as the trade name Striverdi Respimat, Olodaterol is efficacious as a long-acting beta-agonist, which patients self-administer via an easy to use metered dose inhaler. While early statistics from clinical trials of Olodaterol were encouraging, a new set of data was released earlier this week, which only further solidified the effectual and tolerable benefits of this COPD drug.
On September 10, 2013 results from two Phase 3 studies of Olodaterol revealed additional positive results from this formidable COPD treatment. The conclusion from these two 48 week studies, which included over 3,000 patients, showed sizable and significant improvements in the lung function of patients who were dosed with Olodaterol. Patients in the aforementioned studies were administered either a once a day dosage of Olodaterol via the appropriate metered-dose inhaler or “usual care”. The “usual care” included a variety of treatment options, such as inhaled corticosteroids (not Olodaterol), short and long acting anticholinergics, xanthines and beta agonists, which were short acting. The clinical trial participants who were dosed with Olodaterol displayed a rapid onset of action from this drug, oftentimes within the first five minutes after taking this medication. Additionally, patients dispensed the Olodaterol inhaler were successfully able to maintain optimum lung function for longer than a full 24 hour period. The participants who were given Olodaterol experienced such an obvious clinical improvement in their COPD symptoms, and it quickly became apparent that the “usual care” protocol was lacking in efficacy and reliability.
A staggering 24 million patients in the United States suffer from chronic obstructive pulmonary disease, and this patient population is in need of an effectual, safe and tolerable solution. Olodaterol is shaping up to be that much needed solution. Not only have the results from studies of Olodaterol been encouraging, the studies themselves have actually been forward thinking and wellness centered. Boehringer Ingelheim is the first company to included studies to evaluate exercise tolerance in patients with COPD, and compare the data to those patients who were dosed with Olodaterol. By including exercise tolerance as an important benchmark in pertinent data for Olodaterol, Boehringer Ingelheim has created a standard for COPD treatment expectations. The impaired lung function for patients with COPD contributes greatly to their inability to exercise and stay healthy. Patients who find treatments and management techniques to combat the lung hyperinflation that develops during exercise have a distinct advantage to attaining overall good health.
- See more at: http://www.lgmpharma.com/blog/olodaterol-offers-encouraging-results-patients-copd/#sthash.DOjcrGxc.dpuf
Also known as the trade name Striverdi Respimat, Olodaterol is efficacious as a long-acting beta-agonist, which patients self-administer via an easy to use metered dose inhaler. While early statistics from clinical trials of Olodaterol were encouraging, a new set of data was released earlier this week, which only further solidified the effectual and tolerable benefits of this COPD drug.
On September 10, 2013 results from two Phase 3 studies of Olodaterol revealed additional positive results from this formidable COPD treatment. The conclusion from these two 48 week studies, which included over 3,000 patients, showed sizable and significant improvements in the lung function of patients who were dosed with Olodaterol. Patients in the aforementioned studies were administered either a once a day dosage of Olodaterol via the appropriate metered-dose inhaler or “usual care”. The “usual care” included a variety of treatment options, such as inhaled corticosteroids (not Olodaterol), short and long acting anticholinergics, xanthines and beta agonists, which were short acting. The clinical trial participants who were dosed with Olodaterol displayed a rapid onset of action from this drug, oftentimes within the first five minutes after taking this medication. Additionally, patients dispensed the Olodaterol inhaler were successfully able to maintain optimum lung function for longer than a full 24 hour period. The participants who were given Olodaterol experienced such an obvious clinical improvement in their COPD symptoms, and it quickly became apparent that the “usual care” protocol was lacking in efficacy and reliability.
A staggering 24 million patients in the United States suffer from chronic obstructive pulmonary disease, and this patient population is in need of an effectual, safe and tolerable solution. Olodaterol is shaping up to be that much needed solution. Not only have the results from studies of Olodaterol been encouraging, the studies themselves have actually been forward thinking and wellness centered. Boehringer Ingelheim is the first company to included studies to evaluate exercise tolerance in patients with COPD, and compare the data to those patients who were dosed with Olodaterol. By including exercise tolerance as an important benchmark in pertinent data for Olodaterol, Boehringer Ingelheim has created a standard for COPD treatment expectations. The impaired lung function for patients with COPD contributes greatly to their inability to exercise and stay healthy. Patients who find treatments and management techniques to combat the lung hyperinflation that develops during exercise have a distinct advantage to attaining overall good health.
- See more at: http://www.lgmpharma.com/blog/olodaterol-offers-encouraging-results-patients-copd/#sthash.DOjcrGxc.dpuf
Data has demonstrated that Striverdi, a once-daily long-acting beta2 agonist, significantly improved lung function versus placebo and is comparable to improvements shown with the older LABA formoterol. The NHS price for the drug is £26.35 for a 30-day supply.
Boehringer cited Richard Russell at Wexham Park Hospital as saying that the licensing of Stirverdi will be welcomed by clinicians as it provides another option. He added that the trial results showing improvements in lung function “are particularly impressive considering the study design, which allowed participants to continue their usual treatment regimen. This reflects more closely the real-world patient population”.
Significantly, the company is also developing olodaterol in combination with Spiriva, a long-acting muscarinic antagonist. LAMA/LABA combinations provide the convenience of delivering the two major bronchodilator classes.
Adverse effects
Adverse effects generally were rare and mild in clinical studies. Most common, but still affecting no more than 1% of patients, were nasopharyngitis (running nose), dizziness and rash. To judge from the drug's mechanism of action and from experiences with related drugs, hypertension (high blood pressure), tachycardia (fast heartbeat), hypokalaemia (low blood levels of potassium), shaking, etc., might occur in some patients, but these effects have rarely, if at all, been observed in studies.[1]
Interactions
Based on theoretical considerations, co-application of other beta-adrenoceptor agonists, potassium lowering drugs (e. g. corticoids, many diuretics, and theophylline), tricyclic antidepressants, and monoamine oxidase inhibitors could increase the likelihood of adverse effects to occur. Beta blockers, a group of drugs for the treatment of hypertension (high blood pressure) and various conditions of the heart, could reduce the efficacy of olodaterol.[1] Clinical data on the relevance of such interactions are very limited.
Pharmacology
Mechanism of action
Like all beta-adrenoceptor agonists, olodaterol mimics the effect of epinephrine at beta-2 receptors (β₂-receptors) in the lung, which causes the bronchi to relax and reduces their resistance to airflow.[3]
Olodaterol is a nearly full β₂-agonist, having 88% intrinsic activity compared to the gold standard isoprenaline. Its half maximal effective concentration (EC50) is 0.1 nM. It has a higher in vitro selectivity for β₂-receptors than the related drugs formoterol and salmeterol: 241-fold versus β₁- and 2299-fold versus β₃-receptors.[2] The high β₂/β₁ selectivity may account for the apparent lack of tachycardia in clinical trials, which is mediated by β₁-receptors on the heart.
Pharmacokinetics
Once bound to a β₂-receptor, an olodaterol molecule stays there for hours – its dissociation half-life is 17.8 hours –, which allows for once-a-day application of the drug[3] like with indacaterol. Other related compounds generally have a shorter duration of action and have to be applied twice daily (e.g. formoterol, salmeterol). Still others (e. g. salbutamol, fenoterol) have to be applied three or four times a day for continuous action, which can also be an advantage for patients who need to apply β₂-agonists only occasionally, for example in an asthma attack.[8]
History
On 29 January 2013 the U.S. Food and Drug Administration (FDA) Pulmonary-Allergy Drugs Advisory Committee (PADAC) recommended that the clinical data included in the new drug application (NDA) for olodaterol provide substantial evidence of safety and efficacy to support the approval of olodaterol as a once-daily maintenance bronchodilator treatment for airflow obstruction in patients with COPD.[9]
On 18 October 2013 approval of olodaterol in the first three European countries – the United Kingdom, Denmark and Iceland – was announced by the manufacturer.[10]
Figure Chemical structures of salmeterol, formoterol, inda- caterol, and emerging once-daily long-acting β2-agonists
..............................
WO 2004045618 or
Example
a)
- To a solution of 3.6 g 1,1-dimethyl-2-(4-methoxyphenyl)-ethylamine in 100 mL of ethanol at 70 ° C. 7.5 g of (6-benzyloxy-4H-benzo [1,4] oxazin-3-one )-glyoxal added and allowed to stir for 15 minutes. Then within 30 minutes at 10 to 20 ° C. 1 g of sodium borohydride added. It is stirred for one hour, with 10 mL of acetone and stirred for another 30 minutes. The reaction mixture is diluted with 150 mL ethyl acetate, washed with water, dried with sodium sulfate and concentrated. The residue is dissolved in 50 mL of methanol and 100 mL ethyl acetate and acidified with conc. Hydrochloric acid. After addition of 100 mL of diethyl ether, the product precipitates. The crystals are filtered, washed and recrystallized from 50 mL of ethanol. Yield: 7 g (68%; hydrochloride), mp = 232-234 ° C.
b)
- 6.8 g of the above obtained benzyl compound in 125 mL of methanol with the addition of 1 g of palladium on carbon (5%) was hydrogenated at room temperature and normal pressure. The catalyst is filtered and the filtrate was freed from solvent. Recrystallization of the residue in 50 mL of acetone and a little water, a solid is obtained, which is filtered and washed.
Yield: 5.0 g (89%; hydrochloride), mp = 155-160 ° C. - The (R) - and (S)-enantiomers of Example 3 can be obtained from the racemate, for example, by chiral HPLC (for example, column: Chirobiotic T, 250 x 1.22 mm from the company Astec). As the mobile phase, methanol with 0.05% triethylamine and 0.05% acetic acid. Silica gel with a grain size of 5 microns, to which is covalently bound the glycoprotein teicoplanin can reach as column material used. Retention time (R enantiomer) = 40.1 min, retention time (S-enantiomer) = 45.9 min. The two enantiomers can be obtained by this method in the form of free bases. According to the invention of paramount importance is the R enantiomer of Example 3
.................................................
WO 2005111005
Scheme 1.
Scheme 1:
Example 1 6-Hydroxy-8-{(1-hydroxy-2-r2-(4-methoxy-phenyl) - 1, 1-dimethyl-ethylamino]-ethyl)-4H-benzor 41oxazin-3-one - Hvdrochlorid
a) l-(5-benzyloxy-2-hydroxy-3-nitro-phenyl)-ethanone
To a solution of 81.5 g (0.34 mol) l-(5-benzyloxy-2-hydroxy-phenyl)-ethanone in 700 ml of acetic acid are added dropwise under cooling with ice bath, 18 mL of fuming nitric acid, the temperature does not exceed 20 ° C. increases. The reaction mixture is stirred for two hours at room temperature, poured onto ice water and filtered. The product is recrystallized from isopropanol, filtered off and washed with isopropanol and diisopropyl ether. Yield: 69.6 g (72%), mass spectroscopy [M + H] + = 288
b) l-(3-Amino-5-benzyloxy-2-hydroxy-phenyl)-ethanone
69.5 g (242 mmol) of l-(5-benzyloxy-2-hydroxy-3-nitro-phenyl)-ethanone are dissolved in 1.4 L of methanol and in the presence of 14 g of rhodium on carbon (10%) as catalyst at 3 bar room temperature and hydrogenated. Then the catalyst is filtered off and the filtrate concentrated. The residue is reacted further without additional purification. Yield: 60.0 g (96%), R f value = 0.45 (silica gel, dichloromethane).
c) 8-acetyl-6-benzyloxy-4H-benzoπ .4] oxazin-3-one
To 60.0 g (233 mmol) of l-(3-Amino-5-benzyloxy-2-hydroxy-phenyl)-ethanone and 70.0 g (506 mmol) of potassium carbonate while cooling with ice bath, 21.0 ml (258 mmol) of chloroacetyl chloride added dropwise. Then stirred overnight at room temperature and then for 6 hours under reflux. The hot reaction mixture is filtered and then concentrated to about 400 mL and treated with ice water. The precipitate is filtered off, dried and purified by chromatography on a short silica gel column (dichloromethane: methanol = 99:1). The product-containing fractions are concentrated, suspended in isopropanol, diisopropyl ether, and extracted with
Diisopropyl ether. Yield: 34.6 g (50%), mass spectroscopy [M + H] + = 298
d) 6-Benzyloxy-8-(2-chloro-acetyl)-4H-benzoFl, 4] oxazin-3-one 13.8 g (46.0 mmol) of 8-benzyloxy-6-Acetyl-4H-benzo [l, 4] oxazin -3-one and 35.3 g (101.5 mmol) of benzyltrimethylammonium dichloriodat are stirred in 250 mL dichloroethane, 84 mL glacial acetic acid and 14 mL water for 5 hours at 65 ° C. After cooling to room temperature, treated with 5% aqueous sodium hydrogen sulfite solution and stirred for 30 minutes. The precipitated solid is filtered off, washed with water and diethyl ether and dried. Yield: 13.2 g (86%), mass spectroscopy [M + H] + = 330/32.
e) 6-Benzyloxy-8-((R-2-chloro-l-hydroxy-ethyl)-4H-benzori ,41-oxazin-3-one The procedure is analogous to a procedure described in the literature (Org. Lett ., 2002, 4, 4373-4376).
To 13:15 g (39.6 mmol) of 6-benzyloxy-8-(2-chloro-acetyl)-4H-benzo [l, 4] oxazin-3-one and 25.5 mg (0:04 mmol) Cρ * RhCl [(S, S) -TsDPEN] (Cp * = pentamethylcyclopentadienyl and TsDPEN = (lS, 2S)-Np-toluenesulfonyl-l ,2-diphenylethylenediamine) in 40 mL of dimethylformamide at -15 ° C and 8 mL of a mixture of formic acid and triethylamine (molar ratio = 5: 2) dropwise. It is allowed for 5 hours at this temperature, stirring, then 25 mg of catalyst and stirred overnight at -15 ° C. The reaction mixture is mixed with ice water and filtered. The filter residue is dissolved in dichloromethane, dried with sodium sulfate and the solvent evaporated. The residue is recrystallized gel (dichloromethane / methanol gradient) and the product in diethyl ether / diisopropyl ether. Yield: 10.08 g (76%), R f value = 00:28 (on silica gel, dichloromethane ethanol = 50:1).
f) 6-Benzyloxy-8-(R-oxiranyl-4H-benzo ["L4] oxazin-3-one 6.10 g (30.1 mmol) of 6-benzyloxy-8-((R)-2-chloro-l-hydroxy- ethyl)-4H-benzo [l, 4] oxazin-3-one are dissolved in 200 mL of dimethylformamide. added to the solution at 0 ° C with 40 mL of a 2 molar sodium hydroxide solution and stirred at this temperature for 4 hours. the reaction mixture is poured onto ice water, stirred for 15 minutes, and then filtered The solid is washed with water and dried to give 8.60 g (96%), mass spectroscopy [M + H] + = 298..
g) 6-Benyloxy-8-{(R-l-hydroxy-2-r2-(4-methoxy-phenyl)-dimethyl-ll-ethvIaminol-ethyl)-4H-benzo-3-Tl A1oxazin
5.25 g (17.7 mmol) of 6-benzyloxy-8-(R)-oxiranyl-4H-benzo [l, 4] oxazin-3-one and 6.30 g (35.1 mmol) of 2 - (4-methoxy-phenyl 1, 1 - dimethyl-ethyl to be with 21 mL
Of isopropanol and stirred at 135 ° C for 30 minutes under microwave irradiation in a sealed reaction vessel. The solvent is distilled off and the residue chromatographed (alumina, ethyl acetate / methanol gradient). The product thus obtained is purified by recrystallization from a mixture further Diethylether/Diisopropylether-. Yield: 5:33 g (63%), mass spectroscopy [M + H] + = 477 h) 6-Hydroxy-8-{(R)-l-hydroxy-2-[2 - (4-methoxy-phenyl)-l, l-dimethyl-ethylamino] - ethyl}-4H-benzo [1, 4, 1 oxazin-3-one hydrochloride
A suspension of 5:33 g (11.2 mmol) of 6-Benyloxy-8-{(R)-l-hydroxy-2-[2 - (4-methoxy-phenyl)-l, l-dimethyl-ethylamino]-ethyl}-4H -benzo [l, 4] oxazin-3-one in 120 mL of methanol with 0.8 g of palladium on carbon (10%), heated to 50 ° C and hydrogenated at 3 bar hydrogen pressure. Then the catalyst is filtered off and the filtrate concentrated. The residue is dissolved in 20 mL of isopropanol, and 2.5 mL of 5 molar hydrochloric acid in isopropanol. The product is precipitated with 200 mL of diethyl ether, filtered off and dried. Yield: 4.50 g (95%, hydrochloride), mass spectroscopy [M + H] + = 387
...............................
WO 2007020227
........................................
WO 2008090193
or
...............................
Discovery of olodaterol, a novel inhaled beta(2)-adrenoceptor agonist with a 24h bronchodilatory efficacy
Bioorg Med Chem Lett 2010, 20(4): 1410
Bioorg Med Chem Lett 2010, 20(4): 1410
The discovery of the β2-adrenoceptor agonist (R)-4p designated olodaterol is described. The preclinical profile of the compound suggests a bronchoprotective effect over 24 h in humans.
..............
Australia
................................
DUTCH
..............
References
- Striverdi UK Drug Information
- Bouyssou, T.; Casarosa, P.; Naline, E.; Pestel, S.; Konetzki, I.; Devillier, P.; Schnapp, A. (2010). "Pharmacological Characterization of Olodaterol, a Novel Inhaled 2-Adrenoceptor Agonist Exerting a 24-Hour-Long Duration of Action in Preclinical Models". Journal of Pharmacology and Experimental Therapeutics 334 (1): 53–62. doi:10.1124/jpet.110.167007. PMID 20371707.
- Casarosa, P.; Kollak, I.; Kiechle, T.; Ostermann, A.; Schnapp, A.; Kiesling, R.; Pieper, M.; Sieger, P.; Gantner, F. (2011). "Functional and Biochemical Rationales for the 24-Hour-Long Duration of Action of Olodaterol". Journal of Pharmacology and Experimental Therapeutics 337 (3): 600–609. doi:10.1124/jpet.111.179259. PMID 21357659.
- Bouyssou, T.; Hoenke, C.; Rudolf, K.; Lustenberger, P.; Pestel, S.; Sieger, P.; Lotz, R.; Heine, C.; Büttner, F. H.; Schnapp, A.; Konetzki, I. (2010). "Discovery of olodaterol, a novel inhaled β2-adrenoceptor agonist with a 24h bronchodilatory efficacy". Bioorganic & Medicinal Chemistry Letters 20 (4): 1410–1414. doi:10.1016/j.bmcl.2009.12.087. PMID 20096576.
- Joos G, Aumann JL, Coeck C, et al. ATS 2012 Abstract: Comparison of 24-Hour FEV1 Profile for Once-Daily versus Twice-Daily Treatment with Olodaterol, A Novel Long-Acting ß2-Agonist, in Patients with COPD[dead link]
- Van Noord, J. A.; Smeets, J. J.; Drenth, B. M.; Rascher, J.; Pivovarova, A.; Hamilton, A. L.; Cornelissen, P. J. G. (2011). "24-hour Bronchodilation following a single dose of the novel β2-agonist olodaterol in COPD". Pulmonary Pharmacology & Therapeutics 24 (6): 666–672. doi:10.1016/j.pupt.2011.07.006. PMID 21839850.
- van Noord JA, Korducki L, Hamilton AL and Koker P. Four Weeks Once Daily Treatment with BI 1744 CL, a Novel Long-Acting ß2-Agonist, is Effective in COPD Patients. Am. J. Respir. Crit. Care Med. 2009; 179: A6183[dead link]
- Haberfeld, H, ed. (2009). Austria-Codex (in German) (2009/2010 ed.). Vienna: Österreichischer Apothekerverlag. ISBN 3-85200-196-X.
- Hollis A (31 January 2013). "Panel Overwhelmingly Supports Boehringer COPD Drug Striverdi". FDA News/Drug Industry Daily.
- "New once-daily Striverdi (olodaterol) Respimat gains approval in first EU countries". Boehringer-Ingelheim. 18 October 2013.
External links
| WO2002030928A1 | 28 Sep 2001 | 11 Apr 2003 | Boehringer Ingelheim Pharma | Crystalline monohydrate, method for producing the same and the use thereof in the production of a medicament |
| WO2003000265A1 | 8 Jun 2002 | 3 Jan 2003 | Boehringer Ingelheim Pharma | Crystalline anticholinergic, method for its production, and use thereof in the production of a drug |
| WO2004045618A2 * | 11 Nov 2003 | 3 Jun 2004 | Boehringer Ingelheim Pharma | Novel medicaments for the treatment of chronic obstructive pulmonary diseases |
| EP0073505A1 * | 28 Aug 1982 | 9 Mar 1983 | Boehringer Ingelheim Kg | Benzo-heterocycles |
| EP0321864A2 * | 15 Dec 1988 | 28 Jun 1989 | Boehringer Ingelheim Kg | Ammonium compounds, their preparation and use |
| US4460581 | 12 Oct 1982 | 17 Jul 1984 | Boehringer Ingelheim Kg | Antispasmodic agents, antiallergens |
| US4656168 * | 13 Oct 1983 | 7 Apr 1987 | Merck & Co., Inc. | Vision defects; adrenergic blocking and hypotensive agents |
↧
Cebranopadol GRT 6005 セブラノパドール a Potent Analgesic NOP and Opioid Receptor Agonist « New Drug Approvals
Cebranopadol GRT 6005 セブラノパドール a Potent Analgesic NOP and Opioid Receptor Agonist « New Drug Approvals: "Cebranopadol GRT 6005 セブラノパドール a Potent Analgesic NOP and Opioid Receptor Agonist"
'via Blog this'
'via Blog this'
↧
FDA Grants Orphan Designation to Galderma’s Skin Disease Drug Trifarotene « New Drug Approvals
↧
PARP Inhibitor.. Veliparib (ABT-888) 维利帕尼 « New Drug Approvals
↧
↧
BMS-582949 in phase 2 for Treatment of Antipsoriatics , Rheumatoid arthritis « New Drug Approvals
↧
Application of Process Modelling Tools in the Scale-Up of Pharmaceutical Crystallisation Processes

Crystallisations are frequent process steps in the manufacture of active pharmaceutical ingredients (APIs). They are the primary means of intermediate or product formation and separation to achieve the desired purity and form. These unit operations are complex processes which are difficult to control due to the interlinked chemical and physical effects. For example, chemical aspects such as salt and polymorph concerns are in the forefront of process research, but physical effects manifesting themselves on scale-up, due to equipment influences, can be equally important for the successful outcome of a campaign. Several operational parameters, such as temperature or impeller speed, need to be understood and controlled to achieve constant desupersaturation, consistent narrow particle size distribution around the desired mean, minimal attrition, and homogeneous growth conditions. This paper focuses on the equipment influence on crystallisations, relating it to first principles with respect to heat and momentum transfer, analysing it with computational fluid dynamics (CFD), and demonstrating its process impact using examples from recent development work. Dynamic process modelling and CFD are state-of-the-art engineering tools to identify process requirements and match them with equipment capabilities. The work reported here demonstrates how a semiquantitative application of these tools can lead to a controllable, robust process in an existing plant despite the time and resource limitations usually encountered in the industry.
http://pubs.acs.org/doi/full/10.1021/op040013n
Application of Process Modelling Tools in the Scale-Up of Pharmaceutical Crystallisation Processes
GlaxoSmithKline Pharmaceuticals, Old Powder Mills, Tonbridge, Kent, United Kingdom
Org. Proc. Res. Dev., 2004, 8 (6), pp 998–1008
DOI: 10.1021/op040013n
↧
Some thing for your chin………FDA accepts Kythera’s ATX-101 new drug application

FDA accepts Kythera’s ATX-101 new drug application
Kythera Biopharmaceuticals’ new drug application (NDA) for its ATX-101, a submental contouring injectable drug, has been accepted for filing by the US Food and Drug Administration (FDA).
Kythera Biopharmaceuticals’ new drug application (NDA) for its ATX-101, a submental contouring injectable drug, has been accepted for filing by the US Food and Drug Administration (FDA).
According to Kythera Biopharmaceuticals, the ATX-101 NDA will be subject to a standard review and will have a prescription drug user fee act (PDUFA) action date of 13 May 2015. The company submitted the NDA in May 2014.
cas 83-44-3, C24 H40 O4
cas of Na salt….302-95-4
NSC-681065 , NSC 8797
| NAMES | Cholan-24-oic acid, 3,12-dihydroxy-, (3α,5β,12α)- |
- OTHERS
- 5β-Cholan-24-oic acid, 3α,12α-dihydroxy- (8CI); 17β-[1-Methyl-3-carboxypropyl]-etiocholane-3α,12α-diol;
- 3α,12α-Dihydroxy-5β-cholan-24-oic acid;
- 3α,12α-Dihydroxy-5β-cholanic acid;
- 3α,12α-Dihydroxy-5β-cholanoic acid; 3α,12α-Dihydroxycholanic acid;
- 5β-Cholanic acid-3α,12α-diol;
- 5β-Deoxycholic acid; 7-Deoxycholic acid; ATX 101;
- Cholerebic; Cholic acid, deoxy-; Cholorebic; Degalol; Deoxycholatic acid; Deoxycholic acid; Desoxycholic acid; Droxolan; NSC 8797; Pyrochol; Septochol
- Deleted CAS Registry Numbers: 728917-93-9
- University of California, Oakland (Originator)
LA BioMed (Originator) - LICENSE….
Kythera Biopharmaceuticals, Inc.
Rapid removal of body fat is an age-old ideal, and many substances have been claimed to accomplish such results, although few have shown results. ”Mesotherapy”, or the use of injectables for the removal of fat. is not widely accepted among medical practitioners due to safety and efficacy concerns, although homeopathic and cosmetic claims have been made since the 1950′s. Mesotherapy was originally conceived in Europe as a method of utilizing cutaneous injections containing a mixture of compounds for the treatment of local medical and cosmetic conditions. Although mesotherapy was traditionally employed for pain relief, its cosmetic applications, particularly fat and cellulite removal, have recently received attention in the United States. One such reported treatment for localized fat reduction, which was popularized in Brazil and uses injections of phosphatidylcholine, has been erroneously considered synonymous with mesotherapy. Despite its attraction as a purported “fat-dissolving” injection, there is little safety and efficacy data of these cosmetic treatments. See, Rotunda, A.M. and M.
olodney, Dermatologic Surgery 32:, 465-480 (2006) (“Mesotherapy and
Phosphatidy lcholine Injections: Historical Clarification and Review**).
Recently published literature reports that the bile acid, DCA, and salts thereof, have fat removing properties when injected into fatty deposits in vivo. See, WO
2005/1 17900 and WO 2005/1 12942, as well as US2005/0261258; US2005/0267080; US2006/127468; and US20060154906, each of which is incorporated herein by reference in its entirety). Deoxycholate injected into fat tissue degrades fat cells via a cytolytic mechanism. Because deoxycholate injected into fat is rapidly inactivated by exposure to protein and then rapidly returns to the intestinal contents, its effects are spatially contained. As a result of this attenuation effect that confers clinical safety, fat removal typically require 4 – 6 sessions. This localized fat removal without the need for surgery is beneficial not only for therapeutic treatment relating to pathological localized fat deposits (e.g., dyslipidemias incident to medical intervention in the treatment of HIV), but also for cosmetic fat removal without the attendant risk inherent in surgery (e.g., liposuction). See, Rotunda et ai, Dermatol. Surgery 30: 1001-1008 (2004) (“Detergent effects of sodium deoxycholate are a major feature of an injectable phosphatidylcholine formulation used for localized fat dissolution”) and Rotunda et al, J. Am. Acad. Dermatol. (2005 : 973-978) (“”Lipomas treated with subcutaneous deoxycholate injections”), both incorporated herein by reference in their entirety. US Patent Nos. 7,622,130 and
7,754,230 describe using DCA for fat removal.
In addition, many important steroids have a 12- -hydroxy-substituent on the C- ring of the steroid. Such compounds include, by way of example, bile acids such as DCA, cholic acid, lithocholic acid, and the like. Heretofore, such compounds were typically- recovered from bovine and ovine sources which provided a ready source of bile acids on a cost effective basis. However, with the recent discovery that pathogens such as prions can contaminate such sources, alternative methods for the synthesis of bile acids from plant sources or synthetic starting materials have become increasingly important. For example, DCA from animals in New Zealand are a source of bile acids for human use under US regulatory regimes, as long as the animals continue to remain isolated and otherwise free of observable pathogens. Such stringent conditions impose a limitation on the amount of suitable mammalian sourced bile acids and does not preclude the possibility that the bile acid will be free of such pathogens. US Patent Publication No.
8,242,294 relates to DCA containing less than 1 ppt 14C.
ATX-101, sodium deoxycholate for injection, is awaiting for approval in the U.S. for the reduction of localized submental fat. Phase II trials for the treatment of superficial lipomas have been completed at Kythera Biopharmaceuticals and Intendis. Treatment with ATX-101 is expected to significantly reduce the size of or eliminate lipomas and provide an effective non-surgical, minimally invasive treatment option for patients.
Licensed to Kythera from Los Angeles Biomedical Institute at Harbor-UCLA Medical Center in 2007, ATX-101 is also being evaluated by the company for aesthetic applications. Specifically, phase II trials are under way for the reduction of submental fat. In 2010, ATX-101 was licensed to Intendis by Kythera Biopharmaceuticals outside of the U.S. and Canada for the treatment of dermatological disorders. In 2010, the product was licensed by Kythera Biopharmaceuticals to Bayer outside Canada and the U.S., and in 2014, Kythera acquired those same rights from Bayer.
………………………………..
WO 2011075701
Scheme 2
Conversion of Compound 24 to Compound 33:
The hydrogenation of compound 24 on 10.0 g scale using dry 10 % Pd/C (15 wt %) in ethyl acetate (20 parts) was added and applied about 50 psi hydrogen pressure and temperature raised to 70 °C. After reaching temperature 70 °C, observed increase of hydrogen pressure to about 60 psi, at these conditions maintained for 60 h. After 60 hours 0.6% of compound 24 and 2.75% of allylic alcohol were still observed, so further stirred for additional 12 h (observed 0.16% of allylic alcohol and 0.05% of compound 24). After work-up, the reaction provided 9.5g of residue.
Anther hydrogenation reaction on 25 g of compound 24 with above conditions for 76 h provided 24.5 g of residue.
Method A
10% Pd/C (900 mg) was added to a solution of compound 24 (2.0 g, 4.5 mmol) in EtOAc (150 mL) and the resulting slurry was hydrogenated in a Parr apparatus (50 psi) at 50 °C for 16 h. At this point the reaction was determined to be complete by TLC. The mixture was filtered through a small plug of Celite® and the solvent was removed under vacuum, providing compound 33 (1.6 g, 80% yield) as a white solid.
TLC: p-anisaldehyde charring, Rf for 33 = 0.36 and Rf for 25 = 0.32.
TLC mobile phase: 20% – EtOAc in hexanes. 1H NMR (500 MHz, CDC13): δ = 4.67-4.71 (m, 1H), 3.66 (s, 3H), 2.45-2.50 (t, J = 15 Hz, 2H), 2.22-2.40 (m, 1H), 2.01 (s, 3H), 1.69-1.96 (m, 9H), 1.55 (s, 4H), 1.25-1.50 (m, 8H), 1.07-1.19 (m, 2H), 1.01 (s, 6H), 0.84-0.85 (d, J= 7.0 Hz, 3H).
13C NMR (125 MHz, CDC13): δ = 214.4, 174.5, 170.4, 73.6, 58.5, 57.4, 51.3, 46.4, 43.9, 41.2, 38.0, 35.6, 35.5, 35.2, 34.8, 32.0, 31.2, 30.4, 27.4, 26.8, 26.2, 25.9, 24.2, 22.6,
21.2, 18.5,1 1.6,
Mass (m/z) = 447.0 [M+ + 1], 464.0 [M+ + 18].
IR (KBr) = 3445, 2953, 2868, 1731, 1698, 1257, 1029 cm-1.
m.p. =142.2-144.4 °C (from EtOAc/hexanes mixture).
[α]D = +92 (c = 1 % in CHCl3).
ELSD Purity: 96.6%: Retention time = 9.93 (Inertsil ODS 3 V, 250 χ 4.6 mm, 5um, ACN:
0.1 % TFA in water (90: 10)
Method B
A slurry of 10% Pd/C (9 g in 180 mL of ethyl acetate) was added to a solution of compound 24 (36 g, 81 mmol) in EtOAc (720 mL) and the resulting slurry was treated with hydrogen gas (50 psi) at 45-50 °C for 16 h. (A total of 1080 mL of solvent may be used). At this point the reaction was determined to be complete by HPLC (NMT 1% of compound 24). The mixture was filtered through Celite® (10 g) and washed with ethyl acetate (900 mL). The filtrate was concentrated to 50% of its volume via vacuum distillation below 50 °C. To the concentrated solution was added pyridinium
chlorochromate (20.8 g) at 25-35 °C and the mixture was stirred for 2 h at 25-35 °C, when the reaction completed by HPLC (allylic alcohol content is NMT 1%).
The following process can be conducted if compound 24 content is more than 5%. Filter the reaction mass through Celite® (10 g) and wash with ethyl acetate (360 mL). Wash the filtrate with water (3 x 460 mL) and then with saturated brine (360 mL). Dry the organic phase over sodium sulphate (180 g), filter and wash with ethyl acetate (180 mL). Concentrate the volume by 50% via vacuum distillation below 50 °C. Transfer the solution to a clean and dry autoclave. Add slurry of 10% palladium on carbon (9 g in 180 mL of ethyl acetate). Pressurize to 50 psi with hydrogen and stir the reaction mixture at 45-50 °C for 16 h. Upon complete consumption of compound 24 by HPLC (the content of compound 24 being NMT 1%), the reaction mixture was filtered through Celite® (10 g) and the cake was washed with ethyl acetate (900 mL). The solvent was concentrated to dryness via vacuum distillation below 50 °C. Methanol (150 mL) was added and concentrated to dryness via vacuum distillation below 50 °C. Methanol (72 mL) was added to the residue and the mixture was stirred for 15-20 min at 10-15 °C, filtered and the cake was washed with methanol (36 mL). The white solid was dried in a hot air drier at 45-50 °C for 8 h to LOD being NMT 1 % to provide compound 33 (30 g, 83.1 % yield).
Conversion of Compound 33 to Compound 34:
Method A
A THF solution of lithium tri-tert-butoxyaluminum hydride (1 M, 22.4 mL, 22.4 mmol) was added drop wise to a solution of compound 33 (2.5 g, 5.6 mmol) in THF (25 mL) at ambient temperature. After stirring for an additional 4-5 h, the reaction was determined to be complete by TLC. The reaction was quenched by adding aqueous HCl (1 M, 10 mL) and the mixture was diluted with EtOAc (30 mL). The phases were separated and the organic phase was washed sequentially with water (15 mL) and saturated brine solution (10 mL). The organic phase was then dried over anhydrous Na2S04 (3 g) and filtered. The filtrate was concentrated under vacuum and the resulting solid was purified by column chromatography [29 mm x 500 mm (L), 60-120 mesh silica, 50 g], eluting with EtOAc/hexane (2:8) [5 mL fractions, monitored by TLC with p- anisaldehyde charring]. The fractions containing the product were combined and concentrated under vacuum to provide compound 34 (2.3 g, 91%) as a white solid.
TLC: p-anisaldehyde charring, Rf for 34 = 0.45 and Rf for 33 = 0.55.
TLC mobile phase: 30% – EtOAc in hexanes.
1H NMR (500 MHz, CDC13): δ = 4.68-4.73 (m, 1H), 3.98 (s, 1H), 3.66 (s, 3H), 2.34-2.40 (m, 1H), 2.21-2.26 (m, 1H), 2.01 (s, 3H), 1.75-1.89 (m, 6H), 1.39-1.68 (m, 16H), 1.00-1.38 (m, 3H), 0.96-0.97 (d, J= 5.5 Hz, 3H), 0.93 (s, 3H), 0.68 (s, 3H).
13C NMR (125 MHz, CDCI3): δ = 174.5, 170.5, 74.1, 72.9, 51.3, 48.1, 47.2, 46.4, 41.7, 35.8, 34.9, 34.7, 34.0, 33.5, 32.0, 30.9, 30.8, 28.6, 27.3, 26.8, 26.3, 25.9, 23.4, 22.9, 21.3, 17.2, 12.6 Mass (m/z) = 449.0 [M+ + 1], 466.0 [M + 18].
IR ( Br) = 3621, 2938, 2866, 1742, 1730, 1262, 1 162, 1041, cm-1.
m.p = 104.2-107.7 °C (from EtOAc).
[α]D = +56 (c = 1% in CHCl3).
ELSD Purity: 97.0%: Retention time = 12.75 (Inertsil ODS 3V, 250 χ 4.6 mm, 5um, ACN: Water (60:40)
Method B
A THF solution of lithium tri-rert-butoxyaluminum hydride (1 M, 107.6 mL, 107.6 mmol) was added over 1 h to a solution of compound 33 (30.0 g, 67 mmol) in dry THF (300 mL) at 0-5 °C. After stirring for an additional 4 h at 5-10 °C, the reaction was determined to be complete by HPLC (NMT 1% of compound 33). The reaction was cooled to 0-5 °C and quenched by adding 4N HCl (473 mL). The phases were separated. The aqueous layer was extracted with DCM (2 x 225 mL) and the combined organic phase was washed sequentially with water (300 mL) and saturated brine solution (300 mL). The organic phase was then was concentrated to dryness by vacuum distillation below 50 °C. Methanol (150 mL) was added to the residue and concentrated to dryness by vacuum distillation below 50 °C. Water (450 mL) was then added to the residue and the mixture was stirred for 15-20 min., filtered and the cake was washed with water (240 mL). The white solid was dried in a hot air drier at 35-40 °C for 6 h to provide compound 34 (30 g, 99.6%).
Conversion of Compound 34 to crude DCA:
Method A
A solution of LiOH (187 mg, 4.4 mmol) in H20 (2.0 mL) was added to a solution of compound 34 (500 mg, 1.1 1 mmol) in THF (8 mL) and MeOH (8 mL). The resulting mixture was stirred for 3-4 h at 50 °C. Upon complete disappearance of the starting material by TLC, the reaction mixture was concentrated under vacuum. A mixture of water (10 mL) and 3 N HCl (1 mL) were combined and cooled to 0 °C and then added to the crude product. After stirring for 1 h at 0 °C, the precipitated solids were filtered and then washed with water (10 mL) and hexane (20 mL). Drying under vacuum at room temperature provided deoxycholic acid (DCA, 400 mg, 91% yield) as a white solid. TLC: -anisaldehyde charring, Rf for DC A = 0.32 and Rf for 2.1a = 0.82.
TLC mobile phase: 10% – Methanol in DCM.
1H NMR (500 MHz, DMSO): δ = 11.92 (s, 1H), 4.44 (s, 1H), 4.19 (s, 1H), 3.77 (s, 1H), 3.35-3.36 (m, 1H), 2.19-2.21 (m, 1H), 2.08-2.10 (m, 1H), 1.73-1.80 (m, 4H), 1.43- 1.63 (m, 6H), 1.15-1.35 (m, 12H), 0.98-1.05 (m, 2H), 0.89-0.90 (d, J = 6.0 Hz, 3H),
0.83 (s, 3H), 0.58 (s, 3H).
13C NMR (125 MHz, DMSO): δ =174.8, 71.0, 69.9, 47.4, 46.1, 46.0, 41.6, 36.3, 35.6, 35.1, 34.9, 33.8, 32.9, 30.8, 30.7, 30.2, 28.6, 27.1, 27.0, 26.1, 23.5, 23.0, 16.9, 12.4.
Mass (m/z) = 393 [M+, + 1].
IR = 3363, 2933, 2863, 1694, 1453, 1372, 1042, cm-1.
m.p. = 171.4-173.6 °C (from ethanol); 174-176 °C (Alfa Aesar) and 171-174 °C (Aldrich)
[<x]D = +47 (c = 1% in EtOH ), +54° (c = 2% in ethanol) [Alfa Aesar]
ELSD Purity: 99.7%: Retention time = 5.25 (Inertsil ODS 3 V, 250 χ 4.6 mm, 5um, ACN:
0.1% TFA in water (90:10).
Method B
A 20% solution of NaOH (40 g, 270 mmol) in H20 (54 mL) was added to a solution of compound 34 (30 g, 67 mmol) in THF (120 mL) and MeOH (120 mL) at 0-5 °C. The resulting mixture was stirred for 4 h at 25-35 °C. Upon completion of reaction by HPLC (NMT 0.5% of compound 34 and intermediates), the solvent was removed via vacuum distillation below 50 °C. The residue was dissolve in water (300 mL) and washed with DCM (2 x 150 mL). The pH of aqueous layer was adjusted to 1-2 with 2N HCl (~ 173 mL). The solids were filtered, washed thoroughly with water (3 L) and dried by a hot air drier at 70-75 °C until the moisture content is less than 2% to provide deoxycholic acid (DCA, 26 g, 99% yield) as a white solid.
EXAMPLE 9
Deoxycholic acid (DCA) Purification
1. Solvent Selection
Two solvent systems were explored for further purification of DCA: • 10% Hexanes in EtOAc
• DCM
The following experiments have been conducted and the experimental results tabulated below.
* The DCA to be purified was dissolved in a mixture of methanol and DCM and then the methanol was removed by azeotropic distillation. The amount of methanol required to dissolve the crude DCA depends on how pure it is to begin with.
Typical crude material was—75% pure and could be dissolved at reflux using 10% methanol-DCA (by volume) using—20 mL per gram. With purer DCA, the percentage of methanol had to be increased to 15%.
Effective purification was achieved by crystallization of the product from DCM following dissolution in a mixture of methanol and DCM and azeotropic removal of the methanol via atmospheric distillation.
2. Solvent Quantity
Experiments have been conducted using different solvent volumes and the experimental results are tabulated below.
Excellent recoveries and product quality were obtained at all solvent levels.
3. Isolation Temperature
The following experiments have been conducted by varying the isolation temperature and the results are tabulated below:
Higher quality product was obtained when isolation is done at 25-30 °C as compared to 10-15 °C. Purification of DCA in 100 g Scale
The final purification procedure for this step is given below:
Crude DCA (110 g) was dissolved in 10% methanol in DCM (2.5 L) at reflux temperature. To this clear solution 2.5 L of dichloromethane was added at reflux temperature and then about 3.0 L of solvent was distilled at atmospheric pressure (GC analysis of reaction mass supernatant revealed the presence of about 3% of methanol). The reaction slurry was cooled to 20-25 °C and then stirred for 3-4 h. The mixture was filtered and the solids were washed with DCM (300 mL). The product was dried in a hot air oven at 50-55 °C for 6-8 h.
The above dried DCA was added to water (1.0 L) and then 10% sodium hydroxide solution (122 mL) was added resulting in a clear solution. This solution was filtered through 5μ filter paper. The filtrate was diluted with water (2.0 L), and the pH was adjusted to 1— 2 with 2N HCl solution (204 mL). The precipitated solids were stirred for 1 h, filtered and the solids were washed with additional water (7.0 L). After drying in a hot air oven at 70-75 °C for 16-20 h, purified DCA (~ 66 g with more than 99% purity by HPLC RI detection) was obtained as a white solid.
TLC: 7-Anisaldehyde charring, Rf for DCA = 0.32 and Rf for compound 34 = 0.82. Eluent = 10% methanol in DCM. 1H NMR (500 MHz, DMSO): δ = 11.92(s, 1H),4.44(s, 1H), 4.19(s, 1H), 3.77 (s, 1H), 3.36-3.35 (m, 1H), 2.21-2.19 (m, 1H), 2.10-2.08 (m, 1H), 1.80-1.73 (m, 4H), 1.63- 1.43(m, 6H), 1.35-1.15(m, 12H), 1.05-0.98(m, 2H), 0.90-0.89 (d, J = 6.0 Hz, 3H), 0.83 (s, 3H), 0.58 (s, 3H).
1 C NMR (125 MHz, DMSO): δ =174.8, 71.0, 69.9, 47.4, 46.1, 46.0, 41.6, 36.3, 35.6, 35.1, 34.9, 33.8, 32.9, 30.8, 30.7, 30.2, 28.6, 27.1, 27.0, 26.1, 23.5, 23.0, 16.9, 12.4.
Mass (m/z) = 393 [M+, + 1].
IR = 3363, 2933, 2863, 1694, 1453, 1372, 1042, cm-1.
m.p. = 171.4-173.6 °C (from ethanol); 174-176 °C (Alfa Aesar) and 171-174 °C (Aldrich).
Recrystallization of Deoxycholic acid (DC A)
DCA obtained from Method B (26 g) above, was charged into a clean and dry flask. Methanol (65 mL) and DCM (585 mL) were added. The mixture was heated to reflux to obtain a clear solution. DCM (650 mL) was charged to the solution and the solvent was distilled atmospherically until 780 mL of solvent was collected. The mixture was assayed by GC to determine the solvent composition. If the methanol content is more than 2%, add DCM (200 mL) and distill atmospherically until 200 mL of distillate have been collected. (Check for the methanol content by GC). The reaction mixture was cooled over 1-2 h to 20-25 °C and stirred at this temperature for 3-4 h. The product was filtered and washed with DCM (81 mL), dried in a hot air drier at 50-55 °C for 8 h. The purity was determined by HPLC. If single max impurity is more than 0.1%, the above process is repeated.
The dried material from the above was charged in to a clean flask. Water (190 mL) was added and followed by 10% aqueous NaOH (3.18 g in 31.8 mL of water). The solution was filtered through 5μ filter paper and the filtrate was diluted with additional water (380 mL). The pH was adjusted to 1-2 with 2 N HCl (53 mL). The resulting solids was filtered, washed thoroughly with water (1.9 L), and dried in a hot air drier at 70-75 °C until the water content is below 1% to give DCA as a white solid (17 g, % of recovery: 65). EXAMPLE 10
Alternate method of Synthesis and purification of DCA from compound 33
Step la— Hydrogenation of methyl 3a-acetoxy-12-oxo—5fi-chol-9(ll)-en-24-oate (24)
Dry Pd/C (75.0 g, 25 wt %) was added to 24 (300.0 g, 0.7 mol) in EtOAc (7.5 L, 25 vol). The reaction mixture was heated to 45°— 50°C and pressurized to 50 psi of H2. HPLC analysis after 21 hours indicated < 1.0% area under the curve (AUC) of 24 remained; 4.6% AUC of the allylic alcohol impurity 86 and 1 1.1% AUC of the 87 formed. The reaction mixture was cooled to 30° – 35°C, filtered over Hyflo® (300 g) and washed with EtOAc (7.5 L) to remove the catalyst. The resulting filtrate was
concentrated to about 6 L and taken forward without further manipulation (67.8% AUC by HPLC, 5.5% AUC of the allylic alcohol impurity 86 and 13.0% AUC of 87).
Step lb/c – Oxidation of allylic alcohol 86 and 87 and rehydrogenation of 24 to methyl 3a-acetoxy-12-oxo-5fi-cholan-24-oate (33)
Step lb – PCC oxidation of allylic alcohol 86 and 87
A slurry of PCC (149.1 g, 1.03 equiv.) in EtOAc (1.5 L) was added to the 33 solution from above at 20°— 25°C. The reaction was allowed to proceed for 3.5 hours where HPLC analysis showed that < 1% AUC of the allylic alcohol 86 and < 1% AUC of 87 remained. The reaction mixture was filtered over Hyflo® (300 g) and washed with EtOAc (3.0 L). The EtOAc filtrate was washed with deionized (DI) water (2 x 3.6 L) and brine (3.6 L), filtered over Hyflo® (300 g) and washed with EtOAc (3.0 L). The resulting filtrate was concentrated to -7.5 L and taken forward without further manipulation (77.7% AUC by HPLC containing 5.3% AUC of 24).
Step lc— Rehydrogenation of 24 to 33
Powder activated carbon DARCO (60 g, 20 wt %) was added to the crude 33 solution from above containing 24. The resulting slurry was heated to 45°— 50°C for 4 hours, cooled to 30°— 35°C and filtered over Celite®. The filter cake was washed with EtOAc (7.5 L), concentrated to -7.5 L and added to dry Pd/C (60.0 g, 20 wt %). The reaction mixture was heated to 45° – 50°C and pressurized to 50 psi of H2 for 6 hours. HPLC analysis indicated < 1.0% AUC of 24 remained; 1.1% AUC of 86 impurity and < 1.0% AUC of 87 formed. The reaction was deemed complete and cooled to 30° – 35°C, filtered over Celite® and washed with EtOAc (7.5 L). The EtOAc filtrate was concentrated to—5 volumes and azeotroped with MeOH (2 x 4.5 L) back down to—5 volumes. The resulting slurry was diluted with DI water (2.4 L) and maintained at 20-25 °C. The slurry was filtered, washed with DI water (2 x 600 mL) and dried under vacuum at 40° – 50°C to yield 266 g (88%) of 33 (66.2% AUC by HPLC).
Step 2— Synthesis of 34
A solution of 33 (245 g, 0.5 mol) in THF (2.5 L) was cooled to 0° – 5°C and 1 M solution of Li(t-BuO)3A1H (822.9 niL, 1.5 equiv.) was added while maintaining the temperature below 5°C. The reaction mixture was stirred at 5° – 10°C for 22 hours. Reaction may be complete in 2-4 hours. HPLC analysis indicated that the reaction was complete with < 1% of 33 remaining. The reaction was quenched with 4 M HCl (3.7 L) while maintaining the temperature below 20°C. The reaction mixture was extracted with CH2CI2 (2 x 2.5 L) and the combined organic phases were washed with DI water (2 x 2.5 L). The CH2C12 phase was concentrated to afford 300 g (122%) of 34 (73.5% AUC by HPLC). 1H NMPv analysis indicated that 9.7 wt % of THF and 0.8 wt % of CH2C12 remained.
Step 3 – Synthesis of DCA
A NaOH solution (87.6 g, 4 equiv.) in DI water (438.6 mL) was added to a solution of 34 (245 g, 0.5 mol) in MeOH (980 mL) and THF (475 mL) at 0° – 5°C. The reaction mixture was allowed to warm to 20° – 25°C. HPLC analysis showed that the reaction was complete after 1 hour with < 0.5% 34 and < 0.5% of the hydrolysis intermediates remaining. The reaction was diluted with DI water (2.5 L) and
concentrated to—10 volumes. The aqueous solution was washed with CH2C12(2 x 1.3 L) and adjusted to pH 1.7— 2.0 using 2 M HCl (1.6 L). A white slurry formed and was stirred at 20° – 25 °C for 1 hour. The slurry was filtered, washed with DI water (7 x 1 L) and dried under vacuum to yield 195 g (91%) of DCA (82.2% AUC by HPLC).
Step 4 – Purification of DCA
A solution of DCA obtained above (190 g, 0.48 mol) in MeOH (475 mL) and CH2C12 (4275 mL) was heated to 35° – 40°C. The MeOH/CH2Cl2 was distilled out of the mixture while CH2CI2 (4740 mL) was added matching the rate of distillation. Analysis of the solvent composition by Ή NMR indicated 4.5 mol % of MeOH remained relative to CH2C12. The slurry was allowed to cool to 20°— 25°C and held for 16 hours. The solids were isolated by filtration, washed with CH2Cl2 (600 mL) and dried under vacuum to yield 104 g (55%) of DCA (> 99% AUC by HPLC-RID and 98.7% AUC by HPLC- CAD).
The recrystallization was repeated by heating a mixture of DCA (103 g, 0.3 mol) in MeOH (359 mL) and CH2C12 (1751 mL) to 35° – 40°C. The MeOH/CH2Cl2was distilled out of the mixture while CH2CI2 (3760 mL) was added matching the rate of distillation. Analysis of the solvent composition by 1H NMR indicated 4.7 mol % of MeOH remained relative to CH2C12. The slurry was allowed to cool to 20°— 25°C. After 1 hour, the solids were isolated by filtration, washed with CH2CI2 (309 mL) and dried under vacuum to afford 82 g (79%) of DCA (> 99% AUC by HPLC-RID and 99.3% AUC by HPLC-C AD).
To assess the effect of additional purification and reprocessing, the product was recrystallized a third time prior to the normal final water isolation step. The above sample of DCA (80 g, 0.2 mol) in MeOH (240 mL) and CH2C12 (1400 mL) was heated to 35° – 40°C. The MeOH/CH2Cl2 was distilled out of the mixture while CH2C12 (2000 mL) was added matching the rate of distillation. Analysis of the solvent composition by !H NMR indicated 6.7 mol % of MeOH remained relative to CH2C12. The slurry was allowed to cool to 20° – 25°C. After 1 hour, the solids were isolated by filtration, washed with CH2CI2 (240 mL) and dried under vacuum to afford 72 g (89%) of DCA (99.7% AUC by HPLC-CAD).
The sample was slurried in DI water (840 mL) and diluted with a solution of
NaOH (14.0 g) in DI water (140 mL). The resulting solution was filtered over Celite® and washed with DI water (1.4 L). The filtrate was adjusted to pH 1.6 with 2 M HCl (—300 mL) resulting in a white precipitate which was held for 1 hour at 20°— 25°C. The product was isolated by filtration, washed with DI water (9.0°L) and dried under vacuum to afford 63 g (87%) of DCA (99.7% AUC by HPLC-CAD).
SEE MORE IN PATENT
………………………………..
WO 2013044119

Scheme 10
Example 4: Converting Compound 129 To DCA
[0125| In Scheme 1 below, there is provided a scheme for the synthesis and purification of DCA from compound 1.
Scheme 10
A. Conversion of Compound 129 to Compound 130:
Method Al
[0126] 10% Pd/C (900 mg) was added to a solution of compound 129 (2.0 g, 4.5 mmol) in EtOAc (150 mL) and the resulting slurry was hydrogenated in a Parr apparatus (50 psi) at 50 °C for 16 h. At this point the reaction was determined to be complete by TLC. The mixture was filtered through a small plug of Celite® and the solvent was removed under vacuum, providing compound 130 (1.6 g, 80% yield) as a white solid.
TLC: -anisaldehyde charring, Rt for 130 = 0.36. TLC mobile phase: 20% – EtOAc in hexanes.
Ή NMR (500 MHz, CDCL): δ = 4.67-4.71 (m, 1 H), 3.66 (s, 3H), 2.45-2.50 (t, J = 15 Hz, 2H ), 2.22-2,40 (m, 1H), 2.01 (s, 3H). 1 ,69- 1 .96 (m, 9H), 1 ,55 (s, 4H), 1 ,25- 1.50 (m, 8H)5 1.07-1 . 19 (m. 2H), 1 .01 (s, 6H), 0.84-0.85 (d, J = 7.0 Hz, 3H).
13C NMR (125 MHz, CDC13): δ = 214.4, 174.5, 170.4, 73.6, 58,5, 57.4, 51.3, 46,4, 43.9, 41.2, 38.0, 35.6, 35.5, 35.2, 34.8, 32.0, 31 .2, 30.4, 27.4. 26.8, 26.2, 25.9, 24.2, 22.6, 21 .2, 18.5, 1 1.6,.
Mass (m/z) = 447.0 | \! + 1 ], 464.0 [Mf + 18]. IR ( Br) = 3445, 2953, 2868, 1731 , 1698, 1257, 1029 cm“1 , m.p. = 142,2- 144.4 °C (from EtOAc/hexanes mixture). [a]D = +92 (c = l % in CHCl3).
ELSD Purity: 96.6%: Retention time = 9.93 (Inertsil ODS 3V, 250 * 4.6 mm, 5 urn, ACN: 0.1 % TFA in water (90: 10)
Method A2
[0127J A slurry of 10% Pd/C (9 g in 180 mL of ethyl acetate) was added to a solution of compound 129 (36 g, 81 mmol) in EtOAc (720 mL) and the resulting slurry was treated with hydrogen gas (50 psi) at 45-50 °C for 16 h. (A total of 1080 mL of solvent may be used). At this point the reaction was determined to be complete by HPLC (NMT 1 % of compound 129). The mixture was filtered through Cclite® (10 g) and washed with ethyl acetate (900 mL). The filtrate was concentrated to 50% of its volume via vacuum distillation below 50 °C. To the concentrated solution was added pyridinium
chlorochromate (20.8 g) at 25-35 °C and the mixture was stirred for 2 h at 25-35 °C, when the reaction completed by LIPLC (allylic alcohol content is NMT 1 %).
[0128] The following process can be conducted if compound 129 content is more than 5%. Filter the reaction mass through Celite® (10 g) and wash with ethyl acetate (360 mL). Wash the filtrate with water (3 x 460 mL) and then with saturated brine (360 mL). Dry the organic phase over sodium sulphate (180 g), filter and wash with ethyl acetate ( 180 mL). Concentrate the volume by 50% via vacuum distillation below 50 °C. Transfer the solution to a clean and dry autoclave. Add slurry of 10% palladium on carbon (9 g in 1 80 mL of ethyl acetate). Pressurize to 50 psi with hydrogen and stir the reaction mixture at 45-50 °C for 16 h.
[0129] Upon complete consumption of compound 129 by HPLC ( the content of compound 129 being NMT 1 %), the reaction mixture was filtered through Celite® ( 10 g) and the cake was washed with ethyl acetate (900 mL). The solvent was concentrated to dryness via vacuum distillation below 50 °C. Methanol (150 mL) was added and concentrated to dryness via vacuum distillation below 50 °C. Methanol (72 mL) was added to the residue and the mixture was stirred for 15-20 min at 10- 15 °C, filtered and the cake was washed with methanol (36 mL). The white solid was dried in a hot air drier at 45-50 °C for 8 h to LOD being NMT 1% to provide compound 230 (30 g, 83.1 % yield).
B. Conversion of Compound 130 to Compound 1 1.a
Method Bl
[0130J A THF solution of lithium tri-te -butoxyaluminum hydride (1 M. 22.4 mL, 22.4 mmol) was added drop wise to a solution of compound 130 (2.5 g, 5.6 mmol) in THF (25 mL) at ambient temperature. After stirring for an additional 4-5 h, the reaction was determined to be complete by TLC. The reaction was quenched by adding aqueous HQ (1 M, 10 mL) and the mixture was diluted with EtOAc (30 mL). The phases were separated and the organic phase was washed sequentially with water (15 mL) and saturated brine solution (10 mL). The organic phase was then dried over anhydrous Na2SO-i (3 g) and filtered. The filtrate was concentrated under vacuum and the resulting solid was purified by column chromatography [29 mm x 500 mm (L), 60-120 mesh silica, 50 g], eluting with EtOAc/hexane (2:8) [5 mL fractions, monitored by TLC with p- anisaldehyde charring]. The fractions containing the product were combined and concentrated under vacuum to provide compound 131. a (2.3 g, 91 %) as a white solid.
TLC: /7-anisaldehyde charring, Rf for 131. a = 0.45 and Rt for 130 = 0.55. TLC mobile phase: 30% – EtOAc in hexanes.
Ή NMR (500 MHz, CDC13): δ = 4.68-4.73 (m, 1 H), 3.98 (s, 1 H), 3.66 (s, 3H), 2.34-2.40 (m, 1H), 2.21-2.26 (m, 1H), 2.01 (s, 3H), 1.75-1.89 (m, 6H), 1.39-1.68 (m, 16H), 1.00-1.38 (m, 3H), 0.96-0.97 (d, J = 5.5 Hz, 3H), 0.93 (s, 3H), 0.68 (s, 3H).
13C NMR (125 MHz, CDCI3): δ = 174.5, 170.5, 74.1 , 72.9, 51.3, 48.1 , 47.2, 46.4, 41.7, 35.8, 34.9, 34.7, 34.0, 33.5, 32.0, 30.9, 30.8, 28.6, 27.3, 26.8, 26.3, 25.9, 23.4. 22.9, 21.3. 17.2, 12.6
Mass (m/z) = 449.0 [M+ + 1 ], 466.0 [M+ + 18].
IR (KBr) = 3621 , 2938, 2866, 1742, 1730, 1262, 1 162, 1041 , cm4. m.p = 104.2-107.7 °C (from EtOAc).
[<x]D = +56 (c = 1% in CHCI3). ELSD Purity: 97.0%: Retention time = 12.75 (Inertsil ODS 3V, 250 χ 4.6 mm, 5 urn, ACN: Water (60:40)
Method B2
[0131 ] A THF solution of lithium tri-/er?-butoxyaluminum hydride (1 M, 107.6 mL, 107.6 mmol) was added over 1 h to a solution of compound 130 (30.0 g, 67 mmol) in dry THF (300 mL) at 0-5 °C. After stirring for an additional 4 h at 5-10 °C, the reaction was determined to be complete by HPLC (NMT 1% of compound 130). The reaction was cooled to 0-5 °C and quenched by adding 4N HC1 (473 mL). The phases were separated. The aqueous layer was extracted with DCM (2 x 225 mL) and the combined organic phase was washed sequentially with water (300 mL) and saturated brine solution (300 mL). The organic phase was then was concentrated to dryness by vacuum distillation below 50 °C. Methanol (150 mL) was added to the residue and concentrated to dryness by vacuum distillation below 50 °C. Water (450 mL) was then added to the residue and the mixture was stirred for 15-20 min., filtered and the cake was washed with water (240 mL). The white solid was dried in a hot air drier at 35-40 °C for 6 h to provide compound 131.a (30 g, 99.6%).
C. Conversion of Compound 131.a to crude DCA:
[01321 To a solution of 131. a in MeOH (4 vol) and THF (4 vol) was added a solution of NaOH (4.0 equiv) in DI water (5 M) maintaining the temperature below 20 °C. HPLC analysis after 20 hours at 20-25 °C indicated <0.5% AUC of 131.a and the two
intermediates remained. The reaction was deemed complete, diluted with DI water (10 vol) and concentrated to -10 volumes. The sample was azeotroped with 2-MeTHF (2 x 10 vol) and assayed by Ή NMR to indicate MeOH was no longer present. The rich aqueous phase was washed with 2-MeTHF (2 x 10 vol) and assayed by HPLC to indicate 0.3% AUC of the alcohol impurity remained. The aqueous phase was diluted with 2- MeTHF (10 vol ) and adjusted to pH = 1 .7-2.0 using 2 M HC1 (~4 vol ). The phases were separated and the 2-MeTHF phase was washed with DI water (2 x 10 vol). The 2- MeTHF phase was filtered over Celite and the filter cake was washed with 2-MeTHF (2 vol). The 2-MeTHF filtrate was distillated to -10 volumes and azeotroped with ^-heptane containing Statsafe™ 5000 (3 x 10 vol) down to -10 vol. The mixture was assayed by Ή N MR to indicate <5 mol% of 2-MeTHF remained relative to o-heptane. The slurry was held for a minimum of 2 hours at 20-25 °C and filtered. The filter cake was washed with //-heptane (2 x 10 vol) and conditioned under vacuum on the Niitsche filter with N2 for a minimum of 1 hour to afford DCA-crude as white solids. Purity = 94.6% (by HPLC). HPLC analysis for DS-DCA (NMT 5% AUC).
D. Recrystallization of DCA
|0133] DCA-crude was diluted with 2 mol% MeOH in CH2C12 (25 vol) and heated to 35—37 °C for 1 hour. The slurry was allowed to cool to 28-30 °C and filtered. The filter cake was washed with CITC (5 vol) and dried under vacuum at 40 °C to afford DCA. HPLC analysis for DS-DCA (NMT 0.15% AUC).
[0134] DCA was dissolved in 10% DI water/ EtOH (12 vol), polish filtered over Celite and washed with 10% DI water/ EtOH (3 vol). The resulting 15 volume filtrate was added to DI water (30 vol) and a thin white slurry was afforded. The slurry was held for 24 hours, filtered, washed with DI water (20 vol) and dried under vacuum at 40 °C to afford pure DCA. OVI analysis for CH2C12. EtOH. ^-heptane, MeOH and MeTHF was conducted to ensure each solvent was below ICH guideline. KF analysis conducted (NMT 2.0%). Purity = 99.75% (by HPLC). Yield from DCA-crude = 59%.
……………………………
WO 2012174229
In Scheme 1 below, there is provided a scheme for the synthesis and purification of deoxycholic acid from compound 1.
Scheme 1
Conversion of Compound 1 to Compound 2:
[0043] The hydrogenation of compound 1 on 10.0 g scale using dry 10 % Pd/C (15 wt %) in ethyl acetate (20 parts) was added and applied about 50 psi hydrogen pressure and temperature raised to 70 °C. After reaching temperature 70 °C, observed increase of hydrogen pressure to about 60 psi, at these conditions maintained for 60 h. After 60 hours 0.6% of compound 2 and 2.75%> of allylic alcohol were still observed, so further stirred for additional 12 h (observed 0.16% of allylic alcohol and 0.05% of compound 2). After work-up, the reaction provided 9.5 g of residue.
[0044] Anther hydrogenation reaction on 25 g of compound 1 with above conditions for 76 h provided 24.5 g of residue.
Method A
[0045] 10% Pd/C (900 mg) was added to a solution of compound 1 (2.0 g, 4.5 mmol) in EtOAc (150 mL) and the resulting slurry was hydrogenated in a Parr apparatus (50 psi) at 50 °C for 16 h. At this point the reaction was determined to be complete by TLC. The mixture was filtered through a small plug of Celite® and the solvent was removed under vacuum, providing compound 2 (1.6 g, 80%> yield) as a white solid.
TLC: /?-anisaldehyde charring, Rf for 2 TLC mobile phase: 20% – EtOAc in hexanes.
1H NMR (500 MHz, CDC13): δ = 4.67-4.71 (m, 1H), 3.66 (s, 3H), 2.45-2.50 (t, J = 15 Hz, 2H), 2.22-2.40 (m, 1H), 2.01 (s, 3H), 1.69-1.96 (m, 9H), 1.55 (s, 4H), 1.25-1.50 (m, 8H), 1.07-1.19 (m, 2H), 1.01 (s, 6H), 0.84-0.85 (d, J= 7.0 Hz, 3H).
13C NMR (125 MHz, CDC13): δ = 214.4, 174.5, 170.4, 73.6, 58.5, 57.4, 51.3, 46.4, 43.9, 41.2, 38.0, 35.6, 35.5, 35.2, 34.8, 32.0, 31.2, 30.4, 27.4, 26.8, 26.2, 25.9, 24.2, 22.6, 21.2, 18.5,11.6,.
Mass (m/z) = 447.0 [M+ + 1], 464.0 [M+ + 18].
IR (KBr) = 3445, 2953, 2868, 1731, 1698, 1257, 1029 cm“1.
m.p. =142.2-144.4 °C (from EtO Ac/hex anes mixture).
[a]D = +92 (c = 1% in CHC13).
ELSD Purity: 96.6%: Retention time = 9.93 (Inertsil ODS 3V, 250 4.6 mm, 5 urn, ACN: 0.1% TFA in water (90: 10)
Method B
[0046] A slurry of 10%> Pd/C (9 g in 180 mL of ethyl acetate) was added to a solution of compound 1 (36 g, 81 mmol) in EtO Ac (720 mL) and the resulting slurry was treated with hydrogen gas (50 psi) at 45-50 °C for 16 h. (A total of 1080 mL of solvent may be used). At this point the reaction was determined to be complete by HPLC (NMT 1% of compound 1). The mixture was filtered through C elite® (10 g) and washed with ethyl acetate (900 mL). The filtrate was concentrated to 50% of its volume via vacuum distillation below 50 °C. To the concentrated solution was added pyridinium
chlorochromate (20.8 g) at 25-35 °C and the mixture was stirred for 2 h at 25-35 °C, when the reaction completed by HPLC (allylic alcohol content is NMT 1%).
[0047] The following process can be conducted if compound 1 content is more than 5%>. Filter the reaction mass through Celite® (10 g) and wash with ethyl acetate (360 mL). Wash the filtrate with water (3 x 460 mL) and then with saturated brine (360 mL). Dry the organic phase over sodium sulphate (180 g), filter and wash with ethyl acetate (180 mL). Concentrate the volume by 50% via vacuum distillation below 50 °C. Transfer the solution to a clean and dry autoclave. Add slurry of 10% palladium on carbon (9 g in 180 mL of ethyl acetate). Pressurize to 50 psi with hydrogen and stir the reaction mixture at 45-50 °C for 16 h.
[0048] Upon complete consumption of compound 1 by HPLC (the content of compound 1 being NMT 1%), the reaction mixture was filtered through Celite® (10 g) and the cake was washed with ethyl acetate (900 mL). The solvent was concentrated to dryness via vacuum distillation below 50 °C. Methanol (150 mL) was added and concentrated to dryness via vacuum distillation below 50 °C. Methanol (72 mL) was added to the residue and the mixture was stirred for 15-20 min at 10-15 °C, filtered and the cake was washed with methanol (36 mL). The white solid was dried in a hot air drier at 45-50 °C for 8 h to LOD being NMT 1% to provide compound 2 (30 g, 83.1 % yield).
Conversion of Compound 2 to Compound 3:
Method A
[0049] A THF solution of lithium tri-tert-butoxyaluminum hydride (1 M, 22.4 mL, 22.4 mmol) was added drop wise to a solution of compound 2 (2.5 g, 5.6 mmol) in THF (25 mL) at ambient temperature. After stirring for an additional 4-5 h, the reaction was determined to be complete by TLC. The reaction was quenched by adding aqueous HCl (1 M, 10 mL) and the mixture was diluted with EtO Ac (30 mL). The phases were separated and the organic phase was washed sequentially with water (15 mL) and saturated brine solution (10 mL). The organic phase was then dried over anhydrous Na2S04 (3 g) and filtered. The filtrate was concentrated under vacuum and the resulting solid was purified by column chromatography [29 mm x 500 mm (L), 60-120 mesh silica, 50 g], eluting with EtO Ac/hex ane (2:8) [5 mL fractions, monitored by TLC with p- anisaldehyde charring]. The fractions containing the product were combined and concentrated under vacuum to provide compound 3 (2.3 g, 91%) as a white solid.
TLC: /?-anisaldehyde charring, Rf for 3 = 0.45 and Rf for 2 = 0.55.
TLC mobile phase: 30% – EtO Ac in hexanes.
1H NMR (500 MHz, CDC13): δ = 4.68-4.73 (m, 1H), 3.98 (s, 1H), 3.66 (s, 3H), 2.34-2.40 (m, 1H), 2.21-2.26 (m, 1H), 2.01 (s, 3H), 1.75-1.89 (m, 6H), 1.39-1.68 (m, 16H), 1.00-1.38 (m, 3H), 0.96-0.97 (d, J= 5.5 Hz, 3H), 0.93 (s, 3H), 0.68 (s, 3H). ljC NMR (125 MHz, CDC13): δ = 174.5, 170.5, 74.1, 72.9, 51.3, 48.1, 47.2, 46.4, 41.7, 35.8, 34.9, 34.7, 34.0, 33.5, 32.0, 30.9, 30.8, 28.6, 27.3, 26.8, 26.3, 25.9, 23.4, 22.9, 21.3, 17.2, 12.6
Mass (m/z) = 449.0 [M+ + 1], 466.0 [M+ + 18].
IR (KBr) = 3621, 2938, 2866, 1742, 1730, 1262, 1162, 1041, cm“1.
m.p = 104.2-107.7 °C (from EtOAc).
[a]D = +56 (c = 1% in CHC13).
ELSD Purity: 97.0%: Retention time = 12.75 (Inertsil ODS 3V, 250 4.6 mm, 5 urn, ACN: Water (60:40)
Method B
[0050] A THF solution of lithium tri-tert-butoxyaluminum hydride (1 M, 107.6 mL, 107.6 mmol) was added over 1 h to a solution of compound 2 (30.0 g, 67 mmol) in dry THF (300 mL) at 0-5 °C. After stirring for an additional 4 h at 5-10 °C, the reaction was determined to be complete by HPLC (NMT 1% of compound 2). The reaction was cooled to 0-5 °C and quenched by adding 4N HC1 (473 mL). The phases were separated. The aqueous layer was extracted with DCM (2 x 225 mL) and the combined organic phase was washed sequentially with water (300 mL) and saturated brine solution (300 mL). The organic phase was then was concentrated to dryness by vacuum distillation below 50 °C. Methanol (150 mL) was added to the residue and concentrated to dryness by vacuum distillation below 50 °C. Water (450 mL) was then added to the residue and the mixture was stirred for 15-20 min., filtered and the cake was washed with water (240 mL). The white solid was dried in a hot air drier at 35-40 °C for 6 h to provide compound 3 (30 g, 99.6%).
Conversion of Compound 3 to crude DCA:
[0051] To a solution of 3 in MeOH (4 vol) and THF (4 vol) was added a solution of NaOH (4.0 equiv) in DI water (5 M) maintaining the temperature below 20 °C. HPLC analysis after 20 hours at 20-25 °C indicated <0.5% AUC of 3 and the two intermediates remained. The reaction was deemed complete, diluted with DI water (10 vol) and concentrated to ~10 volumes. The sample was azeotroped with 2-MeTHF (2 x 10 vol) and assayed by 1H NMR to indicate MeOH was no longer present. The rich aqueous phase was washed with 2-MeTHF (2 x 10 vol) and assayed by HPLC to indicate 0.3% AUC of the alcohol impurity remained. The aqueous phase was diluted with 2-MeTHF (10 vol) and adjusted to pH = 1.7-2.0 using 2 M HC1 (~4 vol). The phases were separated and the 2-MeTHF phase was washed with DI water (2 x 10 vol). The 2- MeTHF phase was filtered over Celite and the filter cake was washed with 2-MeTHF (2 vol). The 2-MeTHF filtrate was distillated to ~10 volumes and azeotroped with n-heptane containing Statsafe™ 5000 (3 x 10 vol) down to ~10 vol. The mixture was assayed by 1H NMR to indicate <5 mol% of 2-MeTHF remained relative to n-heptane. The slurry was held for a minimum of 2 hours at 20-25 °C and filtered. The filter cake was washed with n-heptane (2 x 10 vol) and conditioned under vacuum on the Nutsche filter with N2 for a minimum of 1 hour to afford DCA-crude as white solids. Purity = 94.6% (by HPLC). HPLC analysis for DS-DCA (NMT 5% AUC).
Recrystallization of Deoxycholic acid (DCA)
[0052] DCA-crude was diluted with 2 mol% MeOH in CH2C12 (25 vol) and heated to 35-37 °C for 1 hour. The slurry was allowed to cool to 28-30 °C and filtered. The filter cake was washed with CH2C12 (5 vol) and dried under vacuum at 40 °C to afford DCA. HPLC analysis for DS-DCA (NMT 0.15% AUC).
[0053] DCA was dissolved in 10% DI water/ EtOH ( 12 vol), polish filtered over Celite and washed with 10% DI water/ EtOH (3 vol). The resulting 15 volume filtrate was added to DI water (30 vol) and a thin white slurry was afforded. The slurry was held for 24 hours, filtered, washed with DI water (20 vol) and dried under vacuum at 40 °C to afford pure DCA. OVI analysis for CH2C12, EtOH, n-heptane, MeOH and MeTHF was conducted to ensure each solvent was below ICH guideline. KF analysis conducted (NMT 2.0%). Purity = 99.75% (by HPLC). Yield from DCA-crude = 59%.
……………………………..
| WO2011075701A2* | Dec 17, 2010 | Jun 23, 2011 | Kythera Biopharmaceuticals, Inc. | Methods for the purification of deoxycholic acid |
| EP0336521B1 * | Apr 7, 1989 | Apr 1, 1992 | Roussel-Uclaf | 9-alpha-hydroxy-17-methylene steroids, process for their preparation and their use in the preparation of corticosteroids |
| US20100179337 * | May 16, 2008 | Jul 15, 2010 | Kythera Biopharmaceuticals, Inc. | Preparation of bile acids and intermediates thereof |
old cut paste
The drug is sodium deoxycholate for injection, code-named ATX-101 was developed for the treatment of lipomas – benign tumors of subcutaneous adipose tissue, as well as other unwanted fatty growths, such as a double chin. This substance, which is a salt of one of the bile acids, emulsifies fats, destroying their excess deposits


ATX-101 (a first-in-class injectable drug being studied for the reduction of localized fat. ATX-101 is a proprietary formulation of deoxycholate a well-studied endogenous compound that is present in the body), a facial injectable drug for the reduction of unwanted fat under the chin, or submental fat. V. Leroy Young, MD, FACS, presented the initial results at the American Society for Aesthetic Plastic Surgery (ASAPS) 45th Annual Aesthetic Meeting in Vancouver, British Columbia, on May 4, 2012.
In August 2010 Bayer Consumer Care AG signed a licensing and development collaboration agreement with KYTHERA, thereby obtaining commercialization rights to ATX-101 outside the US and Canada. KYTHERA and Bayer are collaborating on the development of ATX-101 in Europe.
KYTHERA Biopharmaceuticals Inc. 02 MAR 3013, announced positive interim results from a Phase IIIb multi-center open-label study (ATX-101-11-26) to evaluate the safety and efficacy of ATX-101 an investigational injectable drug for the reduction of unwanted submental fat (SMF) commonly known as double chin. The results presented at the Late Breaking Research Symposium at the 71st American Academy of Dermatology (AAD) Annual Meeting in Miami Beach Fla. found that ATX-101 is well-tolerated and may be effective in reducing SMF by both clinician and patient reported outcome measures. The ATX-101 global clinical development program has enrolled more than 2500 total patients of which more than 1500 have been treated with ATX-101.
“In my practice patients often request a non-surgical way to treat their submental fat or undesirable double chin” said investigator Susan Weinkle MD FAAD a board certified dermatologist and affiliate clinical professor at the University of South Florida. “For these patients double chin is often resistant to diet and exercise. The results of this study suggest that microinjections of ATX-101 can reduce submental fat without worsening skin laxity.”
ATX-101 is a proprietary synthetically-derived formulation of deoxycholic acid (DCA) a naturally-occurring molecule found in the body that aids in fat metabolism. In this open-label Phase IIIb study interim results three months after the last ATX-101 treatment found:
- Reduction of submental fat
- 87 percent of patients achieved at least a one-grade improvement from baseline on the Clinician-Reported Submental Fat Rating Scale (CR-SMFRS)
- Similarly 83 percent of patients achieved at least a one-grade improvement on the Patient-Reported Submental Fat Rating Scale (PR-SMFRS)
- 96 percent of patients had unchanged or improved skin laxity based on the clinician rated Submental Skin Laxity Grading Scale (SMSLG)
- 95 percent of patients were satisfied with treatment based on the Global Post Treatment Satisfaction Scale
- Adverse events were of mild to moderate intensity transient and primarily associated with the treatment area
![]()
Topline results from this study were announced in November 2012. As previously announced 71.3 percent of subjects had at least a one-grade improvement on the CR-SMFRS / PR-SMFRS composite and 14.0 percent had at least a two-grade improvement on the same composite measure.
These results are based on a multicenter 12-month open-label Phase IIIb study conducted at 21 sites across the United States evaluating 165 adults who received injections of ATX-101 for up to six treatments at four-week intervals. Patients received ATX-101 (2 mg/cm2) by subcutaneous microinjections directly into their SMF and were evaluated three months after their last treatment. The study population includes females (77.6 percent) and males (22.4 percent) with a mean age of 47 who report at least moderate SMF and dissatisfaction with the appearance of their chin. All Fitzpatrick Skin Types an industry standard scale to categorize skin tone are represented.
“We are pleased with these ATX-101 study results” said Patricia S. Walker M.D. Ph.D. chief medical officer KYTHERA Biopharmaceuticals Inc. “These results along with efficacy analyses in double-blind placebo-controlled studies support ATX-101 entering the market as potentially the first medical aesthetic drug approved for the reduction of submental fat.”
About ATX-101
ATX-101 is a potential first-in-class injectable drug candidate under clinical investigation for the reduction of unwanted submental fat. ATX-101 is a proprietary formulation of synthetic deoxycholic acid a well-characterized endogenous compound that is present in the body to promote the natural breakdown of dietary fat. ATX-101 is designed to be a locally-injected drug that causes proximal preferential destruction of adipocytes or fat cells with minimal effect on surrounding tissue. Based on clinical trials conducted to date ATX-101 has exhibited significant meaningful and durable results in the reduction of submental fat which commonly presents as an undesirable “double chin.” These results correspond with subject satisfaction measures demonstrating meaningful improvement in perceived chin appearance.
In August 2010 Bayer signed a licensing and collaboration development agreement with KYTHERA thereby obtaining development and commercialization rights to ATX-101 outside of the U.S. and Canada. Bayer recently completed two pivotal Phase III trials of ATX-101 in Europe for the reduction of submental fat. Topline results from these trials were reported in the second quarter of 2012. KYTHERA completed enrollment in its pivotal Phase III clinical program for ATX-101 in more than 1000 subjects randomized to ATX-101 or placebo in 70 centers across the United States and Canada in August 2012. The Company expects to release topline results in mid-2013.
About KYTHERA Biopharmaceuticals Inc.
KYTHERA Biopharmaceuticals Inc. is a clinical-stage biopharmaceutical company focused on the discovery development and commercialization of novel prescription products for the aesthetic medicine market. KYTHERA initiated its pivotal Phase III clinical program for ATX-101 in March 2012 and completed enrollment of more than 1000 patients randomized to ATX-101 or placebo in 70 centers across the U.S. and Canada in August 2012. KYTHERA also maintains an active research interest in hair and fat biology. Find more information at www.kytherabiopharma.com.

THANKS AND REGARD’S
DR ANTHONY MELVIN CRASTO Ph.D
DR ANTHONY MELVIN CRASTO Ph.D
MOBILE-+91 9323115463
GLENMARK SCIENTIST , INDIA
web link
web link
ABOUTME, BRAND ANTHONYCRASTO, COLLECTION OF SITE LINKS,BRAVESITES, GRAVATAR, JIMDO, SKILLPAGES, MIXXT, APNACIRCLE, ZIC ZAC, ZING ME, SCOOP IT, scribd, Stumbleupon, Delicious, pininterest, tumblr, Newsvine, SLIDESHARE, ACADEMIA.EDU, GOOGLE PLUS, FACEBOOK, ISSUU, DIIGO
Congratulations! Your presentation titled “Anthony Cra sto Glenmark scientist, helping millions with websites” has just crossed MILLION views.
アンソニー 安东尼 Энтони 안토니 أنتوني
blogs are ![]()

SCALEUP OF DRUGS, ALL FOR DRUGS ON WEB,
MY CHINA, VIETNAM AND JAPAN BLOGS
ICELAND, RUSSIA, ARAB
GROUPS
you can post articles and will be administered by me on the google group which is very popular across the world
SYNTHETIC ORGANIC CHEMISTRYLinkedIn group

↧
Letrozole boosts fertility in women with PCOS, says study

Letrozole boosts fertility in women with PCOS, says study
Penn State College of Medicine's nationwide study showed that Letrozole resulted in higher birth rates in women with polycystic ovary syndrome (PCOS) than the current preferred infertility treatment drug, Clomiphine citrate. http://www.pharmaceutical-technology.com/news/newsletrozole-boosts-fertility-women-pcos-says-study-4314880?WT.mc_id=DN_News
Penn State College of Medicine's nationwide study showed that Letrozole resulted in higher birth rates in women with polycystic ovary syndrome (PCOS) than the current preferred infertility treatment drug, Clomiphine citrate. http://www.pharmaceutical-technology.com/news/newsletrozole-boosts-fertility-women-pcos-says-study-4314880?WT.mc_id=DN_News
Letrozole (INN, trade name Femara) is an oral non-steroidal aromatase inhibitor for the treatment of hormonally-responsive breast cancer after surgery.

Uses
FDA-approved use
Letrozole is approved by the United States Food and Drug Administration (FDA) for the treatment of local or metastatic breast cancer that is hormone receptor positive or has an unknown receptor status in postmenopausal women.[2]

4-[alpha (4-cyanophenyl)-l-(l,2,4-triazoly)-methyl]- benzonitrile
| Systematic (IUPAC) name | |
|---|---|
| 4,4'-((1H-1,2,4-triazol-1-yl)methylene)dibenzonitrile | |
| Clinical data | |
| Trade names | Femara |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a698004 |
| Licence data | US FDA:link |
| Pregnancy cat. | D (US) |
| Legal status | Schedule VII (CA) POM (UK) ℞-only (US) |
| Routes | Oral |
| Pharmacokinetic data | |
| Bioavailability | 99.9% |
| Protein binding | 60%, mainly to albumin |
| Metabolism | pharmacologically-inactive carbinol metabolite (4,4΄-methanol-bisbenzonitrile)[1] |
| Half-life | 2 days[1] |
| Excretion | Kidneys[1] |
| Identifiers | |
| CAS number | 112809-51-5  |
| ATC code | L02BG04 |
| PubChem | CID 3902 |
| DrugBank | DB01006 |
| ChemSpider | 3765  |
| UNII | 7LKK855W8I  |
| KEGG | D00964  |
| ChEBI | CHEBI:6413  |
| ChEMBL | CHEMBL1444  |
| Chemical data | |
| Formula | C17H11N5 |
| Mol. mass | 285.303 g/mol |
Off-label uses
Letrozole has been used for ovarian stimulation by fertility doctors since 2001 because it has fewer side-effects than clomiphene (Clomid) and less chance of multiple gestation. A Canadian study presented at the American Society of Reproductive Medicine 2005 Conference suggests that letrozole may increase the risk of birth defects. A more detailed ovulation induction follow-up study found that letrozole, compared with a control group of clomiphene, had significantly lower congenital malformations and chromosomal abnormalities at an overall rate of 2.4% (1.2% major malformations) compared with clomiphene 4.8% (3.0% major malformations).[3] Despite this, India banned the usage of letrozole in 2011, citing potential risks to infants.[4] In 2012, an Indian parliamentary committee said that the drug controller office colluded with letrozole's makers to approve the drug for infertility in India and also stated that letrozole's use for infertility was illegal worldwide;[5] however, such off-label uses are legal in many countries such as the US and UK.[6][7]
The anti-estrogen action of letrozole has been shown to be useful in pretreatment for termination of pregnancy, in combination with misoprostol. It can be used in place of mifepristone, which is expensive and unavailable in many countries.[8]
Letrozole is sometimes used as a treatment for gynecomastia, although it is probably most effective at this if caught in an early stage (such as in users of anabolic steroids).[9][10]
Some studies have shown that letrozole can be used to promote spermatogenesis in male patients suffering from nonobstructive azoospermia.[11]
Letrozole has also been shown to delay the fusing of the growth plates in mice.[12] When used in combination with growth hormone, letrozole has been shown effective in one adolescent boy with a short stature.[13]
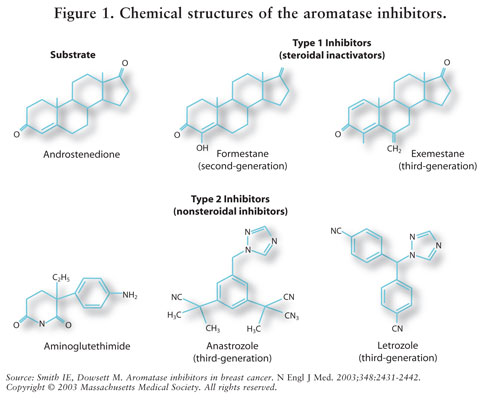
Mechanism of action
Estrogens are produced by the conversion of androgens through the activity of the aromatase enzyme. Estrogens then bind to an estrogen receptor, which causes cells to divide.
Letrozole prevents the aromatase from producing estrogens by competitive, reversible binding to the heme of its cytochrome P450 unit. The action is specific, and letrozole does not reduce production of mineralo- or corticosteroids.
Contraindications
Letrozole is contraindicated in women having a pre-menopausal hormonal status, during pregnancy and lactation.[15]
Adverse effects
Generally, side effects include signs and symptoms of hypoestrogenism. There is concern that long term use may lead to osteoporosis,[2] which is in certain patient populations such as post-menopausal women or osteoporotics, bisphosphonates may also be prescribed.
Interactions
Letrozole inhibits the liver enzyme CYP2A6, and to a lesser extent CYP2C19, in vitro, but no relevant interactions with drugs like cimetidine and warfarin have been observed.[15]
Comparison with tamoxifen
Tamoxifen is also used to treat hormonally-responsive breast cancer, but it does so by interfering with the estrogen receptor. However, letrozole is effective only in post-menopausal women, in whom estrogen is produced predominantly in peripheral tissues (i.e. in adipose tissue, like that of the breast) and a number of sites in the brain.[16] In pre-menopausal women, the main source of estrogen is from the ovaries not the peripheral tissues, and letrozole is ineffective.
In the BIG 1–98 Study, of post-menopausal women with hormonally-responsive breast cancer, letrozole reduced the recurrence of cancer, but did not change survival rate, compared to tamoxifen.[17][18]
Synthesis
...............................................
- Letrozole, chemically known as 4-[alpha (4-cyanophenyl)-1-(1,2,4-triazoly)-methyl]-benzonitrile, and represented by formula (I),is a therapeutically and commercially important non-steroidal aromatase inhibitor, which is widely used for adjuvant treatment of hormonally responsible breast cancer in postmenopausal women. Estrogens are produced by the conversion of androgen through the activity of aromatase enzyme, the suppression of estrogen biosynthesis in peripheral tissues and in the cancer tissue itself can therefore be achieved by specifically inhibiting the aromatase enzyme.
- [0003]1. Bowman et al. were the first to disclose Letrozole in US 4,978,672 , and US 5,352,795 and reported two methods for synthesis of Letrozole, the chemistry for Method-1 is summarized in Scheme-1.
- [0004]The Method-1 for synthesis of Letrozole as disclosed by Bowman et al. in US 4,978,672 , and US 5,352,795 and as summarized in Scheme-1, comprises reaction of alpha-bromo-4 tolunitrile or 4-bromomethyl benzonitrile (II) with 1H-1, 2,4-triazole (III), in a mixture of chloroform and acetonitrile as solvent at reflux temperature for 15 hours to give 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV), which on reaction with 4-flurobenzonitrile (VI) in the presence of potassium t-butoxide and in N, N-dimethylformamaide, gives crude Letrozole (I), which is recrystallized from 95% ethanol or a mixture of ether and ethyl acetate to give pure Letrozole (I):
- [0005]As would be evident from Examples 9, 25, and 26 of US 4,978,672 , and US 5,352,795 , in the step reaction of alpha-bromo-4 tolunitrile or 4-bromomethyl benzonitrile (II) with 1H-1, 2,4-triazole (III), as per Method-1, Scheme-I, in addition to the desired 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV) an appreciable amount of isomeric 4-[1-(1,3,4-triazolyl) methyl]-benzonitrile (V) is also formed in the reaction, which necessitates separation of the two isomers by column chromatography, subsequent to which the separated pure 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV) is reacted with 4-flurobenzonitrile (VI) to give Letrozole. Example 25 of US 4,978,672 , and US 5,352,795 further report that Letrozole obtained after recrystalization from 95% ethanol has a melting point of 181° - 183°C, while Example 26 reports that Letrozole obtained after recrystalization from a mixture of ether and ethyl acetate has a melting point of 184°- 185° C.
- [0006]The major disadvantage and limitation of the Method-1 disclosed in US 4,978,672 , and US 5,352,795 is that it leads to formation of appreciable amounts of the unwanted isomer i.e. 4-[1-(1,3,4-triazolyl) methyl]-benzonitrile (V), calling for tedious chromatographic techniques for its separation from the desired isomer i.e. 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV), which is expected to result in considerable loss and low yield of the desired isomer. Such a method, obviously, cannot be expected to be economically or commercially viable. Further, nowhere in the Specifications and Experimental Descriptions of US 4,978,672 , and US 5,352,795 there is any mention about the yield and purity of Letrozole obtained by the method described therein.
- [0007]The second method, Method-2, reported by Bowman et al. in US 4,978,672 , and US 5,352,795 is summarized in Scheme-II, which comprises of reaction of N-tert.butyl-4-bromo benzamide (1) with n-butyllithium and ethyl formate to give Bis- (4-N-tert.butyl carbamoylphenyl) methanol (2), which on reaction with thionyl chloride gives 4-(alpha-chloro-4'cyanobenzyl)benzonitrile (3). Reaction of 4-(alpha-chloro-4'cyanobenzyl) benzonitrile (3) with 1H-1,2,4-triazole (III) gives Letrozole (I).
- [0008]The major disadvantage and limitation of the Method-2 disclosed in US 4,978,672 , and US 5,352,795 , as evident from Examples 3, 5 and 28, described therein, is that first of all it utilizes corrosive and hazardous n-butyllithium and thionyl chloride; which require special storage, handling and disposal as well as calls for cryogenic temperatures of -60° C and higher temperatures of about 160° C, which collectively renders the method unsafe and industrially and commercially not of particular viability. Further, as in the case of Method-1, nowhere in the Specifications and Experimental Descriptions of US 4,978,672 , and US 5,352,795 there is any mention about the yield and purity of Letrozole obtained by the Method-2 described therein. Furthermore, the reaction of 4-(alpha-chloro-4'cyanobenzyl) benzonitrile (3) with 1,2,4-triazole (III) would most likely result in formation of the corresponding isomer along with the desired Letrozole, which would involve tedious purification techniques for its separation.
- [0009]Improvements over the methods disclosed by Bowman et al. in US 4,978,672 , and US 5,352,795 are the subject matter of the following reports, viz.
- [0010]2. Wadhwa et al. in US 2005/0209294 A1 , recite a method for synthesis of the intermediate 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV), comprising reaction of alpha-bromo-4 tolunitrile or 4-bromomethyl benzonitrile (II) with a salt of 1H-1,2,4-triazole, preferably an alkali metal salt of 1H-1,2,4-triazole (4), in a suitable solvent at a temperature of between 10° to 15° C, followed by crystallization of the isolated product. The chemistry is summarized in Scheme-III.
- [0011]Wadhwa et al. in US 2005/0209294 A1 , while stating that the method disclosed by Bowman et al. in US 4,978,672 , and US 5,352,795 is not selective in that it produces the undesired isomeric 4-[1-(1,3,4-triazolyl) methyl]-benzonitrile (V) in about 50%, which as mentioned hereinbefore requires tedious chromatographic separation techniques for its removal, emphasize that by virtue of utilization of an alkali metal salt of 1H-1, 2,4-triazole (4), the desired 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV) is obtained in >96% selectivity, thereby circumventing the utilization of tedious chromatographic techniques for its purification. Wadhwa et al., further state that the said intermediate i.e. 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV), obtained by their method can be converted to Letrozole of US Pharmacopoeial Quality, through conventional procedure.
- [0012]While the method disclosed by Wadhwa et al. in US 2005/0209294 A1 , reportedly affords the intermediate 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV) in >96% selectivity and further, reportedly does away with chromatographic techniques in its isolation, however, the entire Specification and the Experimental Description given in Example-1 therein, is silent about the actual yield and purity of not only the intermediate 4-[1-(1,2,4-triazolyl) methyl]-benzonitrite (IV) but also that of Letrozole obtained by the method. The industrial or commercial viability of the method, therefore, cannot be commented, in view of insufficient disclosure.
- [0013]3. Kompella et al. in WO 2005/047269 A1 , disclose a method for separation of the Letrozole precursor, 4-[1-(1,2,4-triazolyl)methyl]-benzonitrile (IV) from its isomer, 4-[1-(1,3,4-triazolyl) methyl]-benzonitrile (V), comprising treating a solution of the mixture of the two isomeric compounds (IV) and (V) in dichloromethane or chloroform with isopropylalcohol hydrochloride, followed by addition of isopropyl ether, wherein the hydrochloride salt of the undesired 4-[1-(1,3,4-triazolyl) methyl]-benzonitrile (V) precipitates out, which is removed by filtration. Basification of the filtrate, followed by evaporation of solvent and isolation of the residue from hexane or petroleum ether affords the desired 4-[1-(1,2,4-triazolyl)methyl]-benzonitrile (IV). The method is summarized in Scheme-IV.
- [0014]The required isomer is obtained in 47-61 % yield and a purity of about 99%.
- [0015]4. In another variant of the Method-1 of Bowman et al., an improved regiospecific method disclosed by Patel et al. in US 2006/0128775 A1 for synthesis of Letrozole is summarized in Scheme-V.
- [0016]The method disclosed by Patel et al. in US 2006/0128775 A1 utilizes 4-amino-1, 2,4-triazole (5), instead of 1H-1, 2,4-triazole (III) or an alkali metal salt of 1H-1, 2,4-triazole (4), as utilized by Bowman et al. in US 4,978,672 , and US 5,352,795 and Wadhwa et al. in US 2005/0209294 A1respectively, for reaction with alpha-bromo-4 tolunitrile or 4-bromomethyl benzonitrile (II) to give 4-[(4-amino-1,2,4-triazolium-1-yl)methyl]benzonitrile bromide (6), which on diazotisation leads to the required intermediate, 4-[1-(1,2,4-triazolyl)methyl]-benzonitrile (IV), further reaction of which with 4-flurobenzonitrile (VI) gives crude Letrozole, which is recrystallized from polar or non-polar solvents to give pure Letrozole (I).
- [0017]The method of Patel et al. in US 2006/0128775 A1 , in the first place provides an elegant regiospecific synthesis of Letrozole in that it like the method of Wadhwa et al. in US 2005/0209294 A1 , minimizes the formation of the undesired isomeric 4-[1-(1,3,4-triazolyl) methyl]-benzonitrile (V) and also does away with tedious chromatographic separation techniques.
- [0018]The method of Patel et al. in US 2006/0128775 A1 , albeit, as evident from Example-1, described therein, reportedly gives Letrozole of 99.90% HPLC purity, however, gives Letrozole of the said purity only in an overall yield of 34%, which renders it of not being an particularly economic process. Secondly, the method comprises of an additional step of deamination of the intermediate compound (6), which in turn calls for a diazotization step, through utilization of sodium nitrite, which is hazardous and explosive, more suitable to small scale preparations rather than industrial manufacture. The method, hence, might not be particularly amenable for industrial scale-up and manufacture.
- [0019]5. MacDonald et al. in US 2007/0066831 A1 , report another variant of the methods disclosed by Bowman et al. in US 4,978,672 , and US 5,352,795 and Wadhwa et al. in US 2005/0209294 A1 in that the said method, as summarized in Scheme-VI comprises:
- a) Reaction of alpha-bromo-4 tolunitrile or 4-bromomethyl benzonitrile (II) with an alkali metal salt of 1H-1,2,4-triazole (4), in presence of a solvent selected from the group consisting of diemthylacetamide, N-methyl-2-pyrrolididone, or a mixture thereof, at a temperature of about -20° to 0°C to give 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV);
- b) Extracting the impurities form intermediate compound (IV), in a two phase system, comprising an aqueous phase and a water-immiscible phase; and
- c) Reacting compound (IV) with 4-flurobenzonitrile (VI), in presence of a solvent selected from the group consisting of dimethylformamide, diemthylacetamide, N-methyl-2-pyrrolididone, and tetrahydrofuran or a mixture thereof and a base selected from sodium bis(trimethylsilyl)amide, hexyl lithium, butyl lithium, lithium didsopropylamide, alkoxide or mixtures thereof.
- [0020]US 2007/0066831 A1 further, states that the steps (a) and (b) could be combined together resulting in a one-pot synthesis of Letrozole.
- [0021]In the first place, it might be mentioned herein that the chemistry disclosed by Macdonald et al. in US 2007/0066831 A1 is a nominal variation of the method disclosed by Wadhwa et al. in US 2005/0209294 A1 , in that uses specific solvents such as diemthylacetamide, and N-methyl-2-pyrrolididone for formation of compound (IV) and again utilizes the same solvents for obtaining Letrozole from compound (IV), in addition to use of specific lithium containing bases, most of which are hazardous and expensive, requiring special precautions during storage, handling and disposal.
- [0022]6. In yet another variation, Radhakrishnan et al. in WO 2007/039912 provide a method for synthesis of Letrozole, as summarized in Scheme-VII, which is a one-pot synthesis comprising reaction of compounds (II) and (4) to give compound (IV), which without isolation and on further reaction with compound (VI) gives Letrozole.
- [0023]The major disadvantage with the method is that is still does not obliterate the use of chromatographic separation/purification of Letrozole.
- [0024]7. Haider et al. in WO 2007/054964 A2 provide an improvement, as summarized in Scheme- VIII, over Method-1 disclosed by Bowman et al. in US 4,978,672 and US 5,352.795 . in that the improvement comprises of selective removal of the isomeric 4-[1-(1,3,4-triazolyl)methyl]-benzonitrile (V), formed in the reaction of compound (II) and (III) in isopropanol as solvent, through a method of extraction, which provides the desired 4-[1-(1,2,4-triazolyl)methyl]-benzonitrile (IV), of >99% purity, and relatively free of the isomeric impurity (V).
- [0025]The method of extraction, as taught by Haider et al. in WO 2007/054964 A2 comprises repeated extraction of the reaction medium containing mixture of the desired 4-[1-(1,2,4-triazolyl)methyl]-benzonitrile (IV) and the undesired 4-[1-(1,3,4-triazolyl)methyl]-benzonitrile (V) with water and a water-immiscible solvent to afford the pure 4-[1-(1,2,4-triazolyl)methyl]-benzonitrile (IV) in the organic phase, which is then further converted to Letrozole (I) of >99% purity by conventional methods. Haider et al. also teach a process for conversion of the mixture of the desired 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV) and the undesired 4-[1-(1,3,4-triazolyl)methyl]-benzonitrile (V) to Letrozole, from which the isomeric form of Letrozole i.e. Isoletrozole (9) so formed is removed by repeated crystallization to afford Letrozole (I) of >99% purity. -
- [0026]It might be noted that the method of Haider et al., primarily is one for purification of the intermediate 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV) as well as Letrozole (I) for removal of the corresponding isomeric impurities and as such does not provide any inputs for controlling or minimization of the formation of the isomeric 4-(1-(1,3,4-triazolyl)methyl]-benzonitrile (V) in the reaction. Secondly, the method of extraction as well as purification taught by Haider et al. is tedious, comprising multiple extractions, with multiple solvents and this coupled with the fact that it does not provide any improvement in controlling or minimization of the formation of the isomeric 4-[1-(1,3,4-triazolyl)methyl]-benzonitrile (V) in the reaction, leads to significant losses, thereby resulting in rather low yields of Letrozole (I). The method, therefore, is not of commercial significance.
- [0027]8. Pizzocaro et al. in WO 2007/090464 A1 , a process for preparation of Letrozole (I), as summarized in Scheme-IX, characterized in that it teaches either simultaneous addition of a solution of 4-[1-(1,2,4-triazolyl) methyll-benzonitrile (IV) and a solution of 4-fluorobenzonitrile (VI) in an aprotic dipolar solvent to a solution of an alkali metal alkoxide in the same aprotic dipolar solvent or addition of an unique solution in an aprotic dipolar solvent comprising of compounds (IV) and (VI) to aprotic dipolar solvent, and reacting at a temperature of between -20 to + 40° C.
- [0028]
- [0029]9. Srinivas et al. WO 2007/107733 A1 recite a further variation of Method-1 disclosed by et al. in US 4,978,672 and US 5,352,795 , for synthesis of Letrozole, substantially free from its isomeric impurity, which is summarized in Scheme-X. The method comprises reacting 4-bromomethylbenzonitrile (II), with 1H 1,2,4-triazole (III) in an organic solvent in presence of cesium carbonate and precipitation of 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile (IV), thus formed from the reaction medium using a suitable organic solvent. The intermediate (IV) is further converted to Letrozole by reaction with 4-fluorobenzonitrile (VI) in presence of an organic solvent and silicon amine, which are lithium, sodium, or potassium disilazanes or monosilazane.
- [0030]
- [0031]10. Hasson et al. in US 2007/0112203 A1 , provide a method, as summarized in Scheme-XI, for purification of a mixture containing Letrozole (I) and its isomeric impurity i.e. Isoletrozole (IX), which is an extension of Method-2 disclosed by Bowman et al. in US 4,978,672 and US 5,352,795 . The method takes advantage of the rapid oxidation of Isoletrozole (9) to 4,4'-dicyclobenzophenone (10), in comparison to Letrozole (I), the oxidized compound (10), being easily separable from Letrozole, can be removed by crystallization, affording pure Letrozole. The Letrozole product, in turn is prepared by Method-2 disclosed by Bowman et al. in US 4,978,672 and US 5,352,795 .
- [0032]From the Enabling Disclosures of Hasson et al. in US 2007/0112203 A1 , it could be seen that the method of oxidative purification of Letrozole, does not provide the said Letrozole, free of the Isoletrozole impurity (IX), directly and in fact, about 1 to 4% of Isoletrozole (IX) remains in the product, which is further removed by successive crystallizations to provide Letrozole (I) of 99.9% purity.It is also noted that Letrozole to some extent also undergoes oxidation, albeit slowly, resulting in formation of additional impurities. Removal of such impurities, coupled with the task of removal of Isoletrozole (IX) and 4,4-dicyclobenzophenone (10) results in significant yield loss, rendering the method not particularly attractive, economically.
- [0033]11. Palle et al. in US 2007/0100149 A1 , recite an alternate method for synthesis of Letrozole, as summarized in Scheme-XII.
- [0034]The method of Palle et al. comprises reacting 4,4-(hydroxymethylene)bis benzonitrile (12), in turn obtained from 4,4-dibromobenzophenone (11), with p-toluenesulfonyl chloride to give the corresponding p-tolenesulfonate (13), which on reaction with 1H 1,2,4-triazole (III), gives crude Letrozole, which is further purified by successive chromatography and crystallization.
- [0035]The yield of the p-tolenesulfonate (13), in the key step is only 21%, indicative of formation of large amount of impurities in the said step. Further, the overall yield of Letrozole obtained by the method is only about 14%, which would render the method not viable commercially.
- [0036]12. Friedman et al. in US 2007/0112202 A1 , provide an extension of Method-2 disclosed by Bowman et al. in US 4,978,672 and US 5,352,795 , which is summarized in Scheme-XIII.
- [0037]US 2007/0112202 A1 reports synthesis of Letrozole by the abovementioned method in 54-56% yield and having a HPLC purity 99.4%, which may not suit Pharmacopoeial standards, which suggests that the product obtained requires further purification, which, incidentally, is acknowledged by Friedman et al., who state that single purification using various solvents does not give Letrozole of acceptable purity, and hence multiple purifications are required to achieve the same. Needless to mention, this would result in significant loss of the precious product. Further, the novelty and inventiveness of the method is in question, since Bowman et al. in US 4,978,672 and US 5,352,795 have disclosed the same chemistry earlier.
- [0038]13. Agarwal et al. in WO 2007/074474 A1 recite a synthesis of Letrozole, utilizing novel intermediates, the chemistry of which is summarized in Scheme-XIV.
- [0039]The method is lengthy and the reported overall yield of Letrozole appears to be only 9-11%.
.................................................
The process for preparation of 4-[1-(1,2,4-triazolyl) methyl]-benzonitrile hydrochloride of formula (VII) and Letrozole of formula (I), both having a purity of ≥99% as per the present invention is schematically represented in Scheme-XV.
Reference Example - 3 Preparation of 4-[1-(4-cyanophenyl)-1-(1,2,4-trinzol-1yl)methyl]benzonitrile (Letrozole, I)
- [0101]To a mixture of potassium tertiarybutoxide (635.92 gm; 5.66 mol) and N,N-dimethylformamide (3.75 Lt), under an atmosphere of nitrogen and cooled to a temperature of -20° to -25°C, was added 4-(1H-1,2,4-triazol-1-ylmethyl)benzonitrile hydrochloride (VII, as obtained in Reference Examples 1 or 2; 250 gm; 1.13 mol) within 5 minutes and was stirred for 60 minutes at -20°C to -25°C. To the mixture was added 4-fluoro benzonitrile (VI, 150.9 gm; 1.24 mol) within 5 minutes and the mass agitated for an hour at-20°C to -25°C. After completion of the reaction, pH of the mixture was adjusted to between 6.0 to 6.5 by addition of 50% aqueous hydrochloric acid, maintaining the temperature between -20°C to 0°C. After the addition of the hydrochloric acid solution, the reaction mass was stirred for additional 30 minutes and filtered. To the filtrate was added ethyl acetate and water and the ethyl acetate layer was separated and dried over anhydrous sodium sulfate. The solvent was evaporated under vacuum to give a residual solid amounting to 179 gm (55%) of Letrozole (I), having a purity of 83%.
- [0102]The solid was chromatogaphed over silica gel (60-120 mesh) using n-Hexane and ethyl acetate as eluent to give Letrozole (100.5 gm; 56%), having a purity of 99%.
- [0103]The material (100 gm) was further dissolved in ethyl acetate (1.6 Lt) at 70° to 75°C, and the solution was filtered hot. The filtrate was evaporated under vacuum till the volume was between 200 to 220 ml. The solution was cooled to 0° to 5°C for 4 hours, and the solid filtered, washed with cold ethyl acetate and dried to give Letrozole (I, 95 gm; 95%), having a purity of 99.6%.
Example - 3 Preparation of 4-[1-(4-cyanophenyl)-1-(1,2,4-triazol-1-yl)methyl]benzonitrile (Letrozole, I)
- [0104]To a mixture of potassium tertiarybutoxide (635.92 gm; 5.66 mol) and N,N-dimethylformamide (3.75 Lt). under an atmosphere of nitrogen and cooled to a temperature of -20° to -25°C, was added 4-(1H-1,2,4-triazol-1-ylmethyl)benzonitrile hydrochloride (VII, as obtained in Examples 1 or 2; 250 gm; 1.13 mol) within 5 minutes and was stirred for 60 minutes at -20°C to -25°C. To the mixture was added 4-fluoro benzonitrile (VI, 150.9 gm; 1.24 mol) within 5 minutes and the mass agitated for an hour at -20°C to -25°C. After completion of the reaction, pH of the mixture, was adjusted to between 6.0 to 6.5 by addition of 50% aqueous hydrochloric acid, maintaining the temperature between -20°C to 0°C. After the addition of the hydrochloric acid solution, the reaction mass was stirred for additional 30 minutes and filtered. To the filtrate was added ethyl acetate and water and the ethyl acetate layer was separated and dried over anhydrous sodium sulfate. The solvent was evaporated under vacuum to give a residual solid amounting to 244 gm (75%) of Letrozole (I), having a purity of 99%.
- [0105]The material (244 gm) was further dissolved in ethyl acetate (500 ml) at 70° to 75°C, and the solution was filtered hot. The filtrate was cooled to 0° to 5°C for 4 hours, and the solid filtered, washed with cold ethyl acetate and dried to give Letrozole (I, 221 gm; 98.6%), having a purity of 99.7%.
..................................................
Reference Example - 3 Preparation of4-ll-(4-cyanophenyl)-l-(l,2,4-triazol-l-yl)methyl]benzonitrile (Letrozole, I)
To a mixture of potassium tertiarybutoxide (635.92 gm; 5.66 mol) and N5N- dimethylformamide (3.75 Lt), under an atmosphere of nitrogen and cooled to a temperature of -20° to -25 °C, was added 4-(lH-l,2,4-triazol-l-ylmethyl)benzonitrile hydrochloride (VII, as obtained in Reference Examples 1 or 2; 250 gm; 1.13 mol) within 5 minutes and was stirred for 60 minutes at -20°C to -25°C. To the mixture was added 4-fluoro benzonitrile (VI, 150.9 gm; 1.24 mol) within 5 minutes and the mass agitated for an hour at - 20°C to -25°C. After completion of the reaction, pH of the mixture was adjusted to between 6.0 to 6.5 by addition of 50% aqueous hydrochloric acid, maintaining the temperature between -200C to 0°C. After the addition of the hydrochloric acid solution, the reaction mass was stirred for additional 30 minutes and filtered. To the filtrate was added ethyl acetate and water and the ethyl acetate layer was separated and dried over anhydrous sodium sulfate. The solvent was evaporated under vacuum to give a residual solid amounting to 179 gm (55%) of Letrozole (I), having a purity of 83%.
The solid was chromatogaphed over silica gel (60-120 mesh) using n-Hexane and ethyl acetate as eluent to give Letrozole (100.5 gm; 56%), having a purity of 99%.
The material (100 gm) was further dissolved in ethyl acetate (1.6 Lt) at 70° to 75°C, and the solution was filtered hot. The filtrate was evaporated under vacuum till the volume was between 200 to 220 ml. The solution was cooled to 0° to 5°C for 4 hours, and the solid filtered, washed with cold ethyl acetate and dried to give Letrozole (I, 95 gm; 95%), having a purity of 99.6%. Example - 3 Preparation of4-[l-(4-cyanophenyl)-l-(l,2,4-triazol-l-yl)methyl]benzonitrile (Letrozole, I)
To a mixture of potassium tertiarybutoxide (635.92 gm; 5.66 mol) and N,N- dimethylformamide (3.75 Lt). under an atmosphere of nitrogen and cooled to a temperature of -20° to -25°C, was added 4-(l H-l ,2,4-triazol-l -ylmethyl)benzonitrile hydrochloride (VII. as obtained in Examples 1 or 2; 250 gm; 1.13 mol) within 5 minutes and was stirred for 60 minutes at -20°C to -25°C. To the mixture was added 4-fluoro benzonitrile (VI, 150.9 gm; 1.24 mol) within 5 minutes and the mass agitated for an hour at -20°C to -25°C. After completion of the reaction, pH of the mixtureJwas adjusted to between 6.0 to 6.5 by addition of 50% aqueous hydrochloric acid, maintaining the temperature between -20°C to 0°C. After the addition of the hydrochloric acid solution, the reaction mass was stirred for additional 30 minutes and filtered. To the filtrate was added ethyl acetate and water and the ethyl acetate layer was separated and dried over anhydrous sodium sulfate. The solvent was evaporated under vacuum to give a residual solid amounting to 244 gm (75%) of Letrozole (I), having a purity of 99%.
The material (244 gm) was further dissolved in ethyl acetate (500 ml) at 70° to 75°C, and the solution was filtered hot. The filtrate was cooled to 0° to 5°C for 4 hours, and the solid filtered, washed with cold ethyl acetate and dried to give Letrozole (I, 221 gm; 98.6%), having a purity of 99.7%.
..............................
Aromatase is an enzyme, which effects aromatisation of ring A in the metabolic formation of various steroid hormones. Various cancers, for example, breast cancer is dependent upon circulating steroid hormones, which have an aromatic ring A. Such cancers can be treated by removing the source of ring A aromatised steroid hormones, for example by the combination of oophorectomy and adrenalectomy. An alternative way of obtaining the same effect is by administering a chemical compound, which inhibits the aromatisation of the steroid ring A.
Letrozole is a non-steroidal antineoplastic, claimed to inhibit the aromatase (oestrogen synthase) activity. It is useful in the treatment of advanced breast cancer in postmenopausal women.
The growth of some cancers of the breast are stimulated or maintained by estrogens. Treatment of breast cancer thought to be hormonally responsive (i.e., estrogen and/or progesterone receptor positive or receptor unknown) has included a variety of efforts to decrease estrogen levels (ovariectomy, adrenalectomy, hypophysectomy) or inhibit estrogen effects (antiestrogens and progestational agents). These interventions lead to decreased tumor mass or delayed progression of tumor growth in some women.
In postmenopausal women, estrogens are mainly derived from the action of the aromatase enzyme, which converts adrenal androgens (primarily androstenedioήe and testosterone) to estrone and estradiol. The suppression of estrogen biosynthesis in peripheral tissues and in the cancer tissue itself can therefore be achieved by specifically inhibiting the aromatase enzyme.
Letrozole is a nonsteroidal competitive inhibitor of the aromatase enzyme system; it inhibits the conversion of androgens to estrogens. In adult tumor bearing females, Letrozole is as effective as ovariectomy in reducing uterine weight, elevating serum LH, and causing the regression of estrogen-dependent tumors. In contrast to ovariectomy, treatment with Letrozole does not lead to an increase in serum FSH. Letrozole selectively inhibits gonadal steroidogenesis but has no significant effect on adrenal mineralocorticoid or glucocorticoid synthesis. l Letrozole inhibits the aromatase enzyme by competitively binding to the heme of the cytochrome P450 subunit of the enzyme, resulting in a reduction of estrogen biosynthesis in all tissues. Treatment of women with Letrozole significantly lowers serum estrone, estradiol and estrone sulfate and has not been shown to significantly affect adrenal corticosteroid synthesis, aldosterone synthesis, or synthesis of thyroid hormones. Description of prior art
Synthesis of Letrozole is reported in US Patent No. 4,978,672 and EP 236,940. In the above patents the synthesis of Letrozole starts with 4-bromomethylbenzonitrile (1), which undergoes condensation with 1,2,4-triazole (2) to form 4-[(l,2,4-triazol-l- yl)methyl]benzonitrile (3) as an intermediate. The compound of structural formula (3) is purified by column chromatography to remove 4-[(l,3,4-triazol-l- yl)methyl]benzonitrile (4) and followed by reaction with 4-fluorobenzonitrile (5) to
SCHEME - 1
afford Letrozole (6).
In the above process, the undesired intermediate 4-[(l,3,4-triazol-l- yl)methyl]benzonitrile (4) is formed during the course of the preparation 4-[(l,2,4- triazol-l-yl)methyl]benzonitrile (3) in 10%w/w to 30%w/w. The undesired impurity 4- [(l,3,4-triazol-l-yl)methyl]benzonitrile (4) present with 4-[(l,2,4-triazol-l- yl)methyl]benzonitrile (3), further on reaction with 4-fluorobenzonitrile (5) leads to the formation of another impurity 4-[l-(4-cyanophenyl)-l-(l,3,4-triazol-l- yl)methyl]benzonitrile (7) in approximately same ratio.
To control the formation of impurity of structural formula (7), it is required to make intermediate of structural formula (3) in its pure form. The separation of desired compound from isomeric impurities is of great importance. In basic patents US 4,978,672 and EP 236,940; chromatographic technique is used to isolate intermediate (3) from its mixture with regioisomer (4). Chromatography has its own limitations on commercial scale; it is an expensive and time consuming operation at plant scale, which also consumes lots of solvent and is hazardous for environment.
To overcome the above problems, purification of intermediate (3) is reported in PCT application WO 2005/047269 via the hydrochloride salt formation of the mixture of product (3) along with regioisomer (4). Selective crystallisation of regioisomer as hydrochloride using approximately 8.5 volume diisopropyl ether, filtering the resultant and then isolation of the intermediate (3) as pure product from the filtrate in approximately 60% overall yield. Finally washing the product with hexane or petroleum ether. In above PCT application, highly flammable solvents like diisopropyl ether, hexane and petroleum ether are used in process, which require high level of precautions and are never safe to handle on plant scale.
Another process reported in PCT application WO 2004/076409, discloses the different route to prepare the pure intermediate (3). The said patent discloses a reaction of 4-bromomethylbenzonitrile (1) with 4-amino-l,2,4-triazole (8) to give quaternary ammonium salt (9), which undergoes diazotisation reaction to give 4-[(l,2,4-triazol-l- yl)methyl]benzonitrile (3) in approximately 59% molar yield. The process is complicated and involves lengthy steps and tedious operations. Objects of the invention
It is therefore, an important object of the present invention to provide a process for the preparation of Letrozole which avoids the use of highly flammable solvents and is safe and smooth.. Summary of the invention
To overcome the problems in the use of highly flammable solvents, complicated and lengthy steps and tedious operations; we have opted a simple and novel process for the purification of Letrozole intermediate (3), which is free from its regioisomer (4) and other related impurities.
Purification of intermediate (3) to remove its regioisomer (4) using crystallization method to achieve desired level of purity is unsuccessful. We have planned to go for extraction of intermediate (3) selectively from the mixture with regioisomer (4) in aqueous layer using a suitable solvent.
LETROZOLE
In order to obtain the pure Letrozole (6), we have planned to get intermediate (3) in its pure form and free from its regioisomer (4). For the said purpose, we have used solvent extraction method using suitable solvent system and selectively extract the desired intermediate 4-[(l,2,4-triazol-l-yl)methyl]benzonitrile (3), from a mixture with regioisomer 4-[(l,3,4-triazol-l-yl)methyl]benzonitrile (4). The control of the regioisomer at intermediate level leads its control at the final stage. Therefore, in an embodiment, the present invention relates to Letrozole (6) with its regioisomer 4-[l-(4-cyanophenyl)-l-(l,3,4-triazol-l-yl)methyl]benzonitrile (7), preferably, less than 0.3%w/w, more preferably, less than 0.1%w/w and most preferably, below the quantitation limit.
In another feature, the present invention provides an improved process for the preparation of Letrozole with its regioisomer 4-[l-(4-cyanophenyl)-l-(l,3,4-triazol-l- yl)methyl]benzonitrile (7), preferably, less than 0.3%w/w, more preferably, less tjian 0.1%w/w and most preferably, below the quantitation limit.
In another feature, the present invention provides 4-[(l,2,4-triazol-l- yl)methyl]benzonitrile (3) with its regioisomer 4-[(l,3,4-triazol-l- yl)methyl]benzonitrile (4), preferably, less than 0.3%w/w, more preferably, less than 0.1%w/w and most preferably, below the quantitation limit.
SCHEME - 4
(5)
In yet another feature, the present invention provides an improved process for the preparation of 4-[(l,2,4-triazol-l-yl)methyl]benzonitrile (3) with its regioisomer A- [(l,3,4-triazol-l-yl)methyl]benzonitrile (4), preferably, less than 0.3%w/w, more preferably, less than 0.1%w/w and most preferably, below the quantitation limit.
In order to obtain Letrozole (6) in purer form and free from its regioisomer (7) and other related impurities; intermediate 4-[(l,2,4-triazol-l-yl)methyl]benzonitrile (3) is to be prepared in its pure form, free from its regioisomer 4-[(l,3,4-triazol-l- yl)methyl]benzonitrile (4) and other related impurities. Thus, the main aspect of the present invention relates to the preparation of Letrozole (6) with its regioisomer (7) preferably less than 0.3%, more preferably less than 0.1% and most preferably below quantitation limit. For this purpose intermediate 4-[(l,2,4-triazol-l- yl)methyl]benzonitrile (3) is required to be of the same purity level. Another aspect of the present invention relates to the preparation of 4-[(l,2,4-triazol-l- yl)methyl]benzonitrile (3) with its regioisomer 4-[(l,3,4-triazol-l- yl)methyl]benzonitrile (4) preferably less than 0.3%, more preferably less than 0.1% and most preferably below quantitation limit.
It has been also found that during the preparation of Letrozole intermediate A- [(l,2,4-triazol-l-yl)methyl]benzonitrile (3) another impurity is formed, which is characterized as the quaternary salt (10). To control the formation of this quaternary salt, mole ratio of 1,2,4-triazole is optimized preferably from 1.5 mole to 4.4 mole equivalents and more preferably to 3.0 mole equivalents with respect to A- bromomethylbenzonitrile (1). Thus, another aspect of the present invention relates to the preparation of Letrozole (6) with quaternary salt (10) preferably less than 0.1% and more preferably below quantitation limit. Another important aspect of the present invention relates to the preparation of 4-
[(l,2,4-triazol-l-yl)methyl]benzonitrile (3) in the pure form, free from its regioisomer 4-[(l,3,4-triazol-l-yl)methyl]benzonitrile (4). The purification of 4-[(l,2,4-triazol-l- yl)methyl]-benzonilrilc (3) takes place by its selective extraction from a mixture with its regioisomer 4-[(l,3,4-triazol-l-yl)methyl]benzonitrile (4) by using suitable solvents and/or mixture of solvents.
According to another aspect of the present invention Letrozole intermediate A- [(l,2,4-triazol-l-yl)methyl]benzonitrile (3) is prepared with its regioisomer 4-[(l,3,4- triazol-l-yl)methyl]benzonitrile (4) less than 30% and followed by the preparation of Letrozole enriched with its regioisomer (7), which is removed by using crystallisation method using suitable solvent system.
Example - 3
4-[(l,2,4-triazol-l-yl)methyl]benzonitrile (S) with a mixture of 4-[(l,3,4-triazol-l- yl)methyl]benzonitrile (4) To a 250 mL three neck R. B. Flask fitted with a reflux condenser and a thermometer pocket, isopropanol (37.5 mL), p-cyanobenzylbromide (25 g), 1,2,4- triazole (26.4 g) and potassium carbonate (52.8 g) were charged to the reaction mixture at RT with stirring. The reaction mixture was heated to 60-65 0C for 1.0 hr. The progress of reaction was monitored over TLC for the absence of p- cyanobenzylbromide. After completion, the reaction mixture was cooled down to RT and water (100 mL) was added to the reaction mixture and reaction mass was transferred to a one lit R. B. Flask containing water (275 mL). Cone. HCl (50 mL) was added very slowly to the reaction mass to adjust pH 7 - 8. The reaction mixture was extracted from dichloromethane (250 mL). Dichloromethane layer was distilled out at 50 0C giving 21.0 gm viscous oily residue. The residue is crystallized from a mixture of IPA: Cyclohexane (1:10) to give 18 g of 4-[(l,2,4-triazol-l-yl)methyl]benzonitrile (3) with a mixture of its regioisomer (4). HPLC Purity ~ 85%; Regioisomer ~ 13%. Example - 4 4-[l-(4-cyanophenyl)-l-(l,2,4-triazol-l-yl)methyl]benzonitrile (6) & 4-[l-(4- cyanophenyl)-l-(l,3,4-triazol-l-yl)methyl]benzonitrile (7)
In a 250 mL three neck flask, equipped with thermometer pocket, mechanical stirrer and a guard tube, THF (50 mL) was charged at room temperature. Potassium tert-butoxide (12.3 gm) is added in small portions in 30 minutes. The solution was cooled to -15 0C and a solution of product from example-3 (10 g) and p- fluorobenzonitrile (8.5 g) in THF (50 mL) is added very slowly to the reaction mixture in 4-5 hrs. Stir the reaction mixture at same temperature for 3 hrs. Progress of the reaction is monitored on TLC. Dichloromethane (200 mL) is added to the reaction mixture followed by the addition of acetic acid (7 g). Reaction mixture is added to another flask containing water (220 mL). pH of the reaction mixture is adjusted to 7-8 by addition of 5% sodium bicarbonate solution (180 mL). Dichloromethane layer is washed with water (200 mL), separated, filtered through hyfiow bed and distilled at a temperature below 50 0C. The residue obtained was crystallized from Isopropanol (20 mL) to get the solid product (4.9 g). HPLC Purity: 89.6%. Regioisomer: 7.41%. Example - 5
Removal of regioisomer (7) from Letrozole (6)
To a 250 mL R. B. Flask crude Letrozole (4.5 g) was charged in methanol (115 mL) and heated to 60 0C to dissolve completely and get clear solution. Methanol (approx. 100 mL) was distilled out and the solution was cooled to 25 - 30 0C, and was stirred for 2 hrs at this temperature. Solid product was filtered and washed with methanol (10 mL x 2) to get solid product, which was dried in vacuum to get 3.5 gm of product.
HPLC Purity: 96.33%, Regioisomer: 3.4%
Using the same purification method, desired purity of Letrozole had been achieved containing acceptable level of regioisomer (7).
Following the above purification from methanol repeatedly, the Letrozole may be prepared with the desired limit of its regioisomer (7).
References
- 003330 Letrozole
- Drugs.com: monograph for letrozole. It is also used for ovarian cancer patients after they have completed chemotherapy.
- Tulandi T, Martin J, Al-Fadhli R, et al. (June 2006). "Congenital malformations among 911 newborns conceived after infertility treatment with letrozole or clomiphene citrate". Fertility and Sterility 85 (6): 1761–5. doi:10.1016/j.fertnstert.2006.03.014. PMID 16650422.
- Sinha, Kounteya (18 October 2011). "Finally, expert panel bans fertility drug Letrozole". The Times of India. Retrieved 14 November 2011.
- "House panel to govt: Punish those guilty of approving Letrozole". The Times of India. 10 April 2007. Retrieved 9 May 2012.
- Chen DT, Wynia MK, Moloney RM, Alexander GC (2009). "Physician knowledge of the FDA-approved indications of commonly prescribed drugs: results of a national survey". Pharmacoepidemiology and Drug Safety 18 (11): 1–7. doi:10.1002/pds.1825. PMID 19697444.
- "GMC | Good practice in prescribing medicines – guidance for doctors". Gmc-uk.org. 16 February 2007. Retrieved 21 November 2011.
- Vivian Chi Yan Lee, Ernest Hung Yu Ng, William Shu Biu Yeung, Pak Chung Ho (2011). "Misoprostol With or Without Letrozole Pretreatment for Termination of Pregnancy". Ob Gyn. 117 (2, Part 1): 317–323. doi:10.1097/AOG.0b013e3182073fbf.
- Santen, R. J.; Brodie, H.; Simpson, E. R.; Siiteri, P. K.; Brodie, A. (2009). "History of Aromatase: Saga of an Important Biological Mediator and Therapeutic Target". Endocrine Reviews 30 (4): 343–375. doi:10.1210/er.2008-0016. PMID 19389994.
- "Gynecomastia and Letrozole". GYNECOMASTIA-GYNO.COM: ...a resource for gynecomastia sufferers... 16 December 2008. Archived from the original on 26 June 2010. Retrieved 26 April 2012.
- Geneviève Patry, Keith Jarvi, Ethan D. Grober, Kirk C. Lo (August 2009). "Use of the aromatase inhibitor letrozole to treat male infertility". Fertility and Sterility 92 (2): 829.e1–829.e2. doi:10.1016/j.fertnstert.2009.05.014.
- R Eshet, G Maor, T Ben Ari, M Ben Eliezer, G Gat-Yablonski, M Phillip (2004). "The aromatase inhibitor letrozole increases epiphyseal growth plate height and tibial length in peripubertal male mice". Journal of Endocrinology 182 (1): 165–172. doi:10.1677/joe.0.1820165. PMID 15225141.
- Ping Zhou MD, Bina Shah MD, Kris Prasad PhD, Raphael David MD (2005). "Letrozole Significantly Improves Growth Potential in a Pubertal Boy With Growth Hormone Deficiency". Journal of the American Academy of Pediatrics 115 (2): 245–248. doi:10.1542/peds.2004-1536. PMID 15653791.
- Endometriosis ESHRE abstract
- Haberfeld, H, ed. (2009). Austria-Codex (in German) (2009/2010 ed.). Vienna: Österreichischer Apothekerverlag. ISBN 3-85200-196-X.
- Simpson ER (2003). "Sources of estrogen and their importance". The Journal of Steroid Biochemistry and Molecular Biology 86 (3–5): 225–30. doi:10.1016/S0960-0760(03)00360-1. PMID 14623515.
- Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer, the BIG 1–98 Collaborative Group, N Engl J Med, 361:766, 2009 Aug 20
- 32nd Annual San Antonio Breast Cancer Symposium
- Lang, M; Batzl, C; Furet, P; Bowman, R; Hausler, A; Bhatnagar, A (1993). "Structure-activity relationships and binding model of novel aromatase inhibitors". The Journal of Steroid Biochemistry and Molecular Biology 44 (4–6): 421–8. doi:10.1016/0960-0760(93)90245-R. PMID 8476755.
External links
↧
↧
Anthony crasto’s blog New drug approvals touches 3 lakh views…….Helping millions « New Drug Approvals
Anthony crasto’s blog New drug approvals touches 3 lakh views…….Helping millions « New Drug Approvals: "Anthony crasto’s blog New drug approvals touches 3 lakh views…….Helping millions"
'via Blog this'
'via Blog this'
↧
Anthony crasto's blog New drug approvals touches 3 lakh views.......Helping millions

link is http://newdrugapprovals.org/
All about Drugs, live, by DR ANTHONY MELVIN CRASTO, Worlddrugtracker, Helping millions, 7 million hits on google, pushing boundaries, one lakh plus connections worldwide, 3 lakh plus VIEWS on this blog in 193 countries![]()

DR ANTHONY MELVIN CRASTO Ph.D
amcrasto@gmail.com
MOBILE-+91 9323115463
GLENMARK SCIENTIST , INDIA
web link
web link
ABOUTME, BRAND ANTHONYCRASTO, COLLECTION OF SITE LINKS, BRAVESITES, GRAVATAR, JIMDO, SKILLPAGES, MIXXT, APNACIRCLE, ZIC ZAC, ZING ME, SCOOP IT, scribd, Stumbleupon, Delicious, pinnterest, tumblr, Newsvine, SLIDESHARE, ACADEMIA.EDU, GOOGLE PLUS, FACEBOOK, TWITTER, ISSUU, DIIGO, YOLASITE, Authorstream, Symbaloo, ISSUU, BLOGLOVIN, FRIENDFEED, NEWSVINE
Congratulations! Your presentation titled "Anthony Cra sto Glenmark scientist, helping millions with websites" has just crossed MILLION views.
アンソニー 安东尼 Энтони 안토니 أنتوني
blogs are ![]()

New Drug Approvals, ALL ABOUT DRUGS, WORLD DRUG TRACKER
MEDICINAL CHEM INTERNATIONAL, DRUG SYN INTERNATIONAL
SCALEUP OF DRUGS, ALL FOR DRUGS ON WEB,
MEDICINAL CHEM INTERNATIONAL, DRUG SYN INTERNATIONAL
SCALEUP OF DRUGS, ALL FOR DRUGS ON WEB,
MY CHINA, VIETNAM AND JAPAN BLOGS
http://me.zing.vn/u/amcrasto
ICELAND, RUSSIA, ARAB
BOBRDOBR, BLAND ICELAND, 100zakladok, adfty
GROUPS
you can post articles and will be administered by me on the google group which is very popular across the world
OPD GROUPSPACES, SCOOP OCI, organic-process-development GOOGLE, TVINX, MENDELEY WDT, SCIPEOPLE OPD, EPERNICUS OPD, SYNTHETIC ORGANIC CHEMISTRYLinkedIn group, DIIGO OPD, LINKEDIN OPD, WDT LINKEDIN, WDTI ZING
ICELAND, RUSSIA, ARAB
BOBRDOBR, BLAND ICELAND, 100zakladok, adfty
GROUPS
you can post articles and will be administered by me on the google group which is very popular across the world
OPD GROUPSPACES, SCOOP OCI, organic-process-development GOOGLE, TVINX, MENDELEY WDT, SCIPEOPLE OPD, EPERNICUS OPD, SYNTHETIC ORGANIC CHEMISTRYLinkedIn group, DIIGO OPD, LINKEDIN OPD, WDT LINKEDIN, WDTI ZING

↧
Organo-Iodine Compounds Analysis by the 5975-SMB GC-MS with Cold EI
Organo-Iodine Compounds Analysis by the 5975-SMB GC-MS with Cold EI
Aviv Amirav, Professor of Chemistry at Tel Aviv University and Director - Aviv Analytical
Larisa Panz Ph.D., Ksenia Kulbitski and Professor Mark Gendelman, Schulich Faculty of Chemistry at the Technion Haifa Israel.
read at
↧
Decernotinib ... JAK inhibitor for the treatment of autoimmune and inflammatory diseases, including rheumatoid arthritis.
Decernotinib
Decernotinib
N2-[2-(1H-Pyrrolo[2,3-b]pyridin-3-yl)pyrimidin-4-yl]-N-(2,2,2-trifluoroethyl)-D-isovalinamide
(R)-2-(2-(lH-pyrrolo[2,3-b]pyridin-3-yl)pyrimidin-4-ylamino)-2-methyl-N-(2,2,2- trifluoroethyl)butanamide
Vertex Pharmaceuticals Inc
UNII-MZK2GP0RHK, VX-509, VRT-831509, cas 944842-54-0
Molecular Formula: C18H19F3N6O
Molecular Weight: 392.37827
In phase 3 for the treatment of autoimmune and inflammatory diseases, including rheumatoid arthritis.
The Janus kinases (JAK) are a family of tyrosine kinases consisting of JAK1, JAK2, JAK3, and TYK2. The JAKs play a critical role in cytokine signaling. The down-stream substrates of the JAK family of kinases include the signal transducer and activator of transcription (STAT) proteins. JAK/STAT signaling has been implicated in the mediation of many abnormal immune responses such as psoriasis. Moreover, JAK kinases represent an established therapeutic target for this disease.
For example, JAK kinases are an established therapeutic target for treating psoriasis. Stump K. L., et al., Arthritis Res. Ther. (201 1) 13:R68; Fridman J.S., et al., J Immunol. (2010) 184:5298-5307; West K., Curr. Op. Investig. Drugs (2009) 10:491-504; Kremer J. M. et al., Arthritis Rheumatism (2009) 60(7):1895- 1905; Xiong, W. et al., Ther Adv Musculoskelet Dis. (201 1) 3(5): 255-266; Panes, J. et al. 19th Ann. Eur. Gastroenterology Week (Oct 22-26, 2011) Stockholm, SE, PI 456; and Drugs in R & D "Tofacitinib" (2010) 10(4):271-84.
Compounds described as kinase inhibitors, particularly the JAK family kinases, are disclosed in WO 2005/095400 and WO 2007/084557. Also disclosed in these publications are processes and intermediates for preparing these compounds
Decernotinib ( VX-509 ) is an oral selective JAK3 inhibitor being evaluated for the treatment of rheumatoid arthritis ( RA ). This was a 24-week, randomized, placebo-controlled, double-blind, phase 2 study of four dosing regimens of Decernotinib, administered to patients with RA with inadequate response to Methotrexate ( MTX ).
The aim of the study was to assess the efficacy and safety of four dosing regimens of VX-509 administered to patients with rheumatoid arthritis on stable background Methotrexate therapy.
Patients with active rheumatoid arthritis ( C-reactive protein [ CRP ] greater than ULN, greater than or equal to 6 swollen joints [ of 66 ], and greater than or equal to 6 tender joints [ of 68 ] ) taking stable doses of MTX were randomized 1:1:1:1:1 to receive placebo or one of four dosing regimens of Decernotinib ( 100 mg QD, 150 mg QD, 200 mg QD, or 100 mg BID ) for a duration of 24 weeks.
The primary efficacy endpoints at week 12 were met and have previously been reported; 24-week efficacy and safety results are now reported.
A total of 358 patients were randomized and received greater than or equal to 1 dose of study drug; 81% of patients were female, with a mean age of 53 years.
At baseline, the mean tender joint count was 23.8, the mean swollen joint count was 16.1, and the average disease duration was 7.3 years.
At baseline, the mean tender joint count was 23.8, the mean swollen joint count was 16.1, and the average disease duration was 7.3 years.
After 24 weeks of treatment the proportion of patients achieving ACR20, ACR50, ACR70, DAS28 ( CRP ) less than 2.6 and DAS28 ( ESR ) less than 2.6 and the decrease from baseline in DAS28 ( CRP ) were statistically significantly greater in each of the Decernotinib dose groups than in the placebo group.
Over 24 weeks, the percentage of patients with any adverse event was higher in the Decernotinib group ( all Decernotinib dose groups combined ) ( 59.9% ) relative to placebo ( 42.3% ) and led to study discontinuation in 9.1% and 8.5% of patients in the Decernotinib and placebo groups, respectively.
The most common adverse reactions in the Decernotinib group were headache ( 8.7% ), hypercholesterolemia ( 5.2% ), and diarrhea ( 4.5% ).
Serious adverse reactions occurred in similar proportions of patients receiving Decernotinib ( 7.3% ) or placebo ( 5.6% ), but there were more serious infections in the Decernotinib group ( 3.5% ) compared with placebo ( 1.4% ).
Through 24 weeks there were two serious adverse effects that resulted in death; one was cardiac failure in the Decernotinib 100 mg BID group ( previously reported ) and one was pancytopenia in a patient with pneumonia in the Decernotinib 200 mg QD group.
Elevations in transaminase levels and decreases in median neutrophil and lymphocyte counts were observed in the Decernotinib groups and were generally mild.
The most common adverse reactions in the Decernotinib group were headache ( 8.7% ), hypercholesterolemia ( 5.2% ), and diarrhea ( 4.5% ).
Serious adverse reactions occurred in similar proportions of patients receiving Decernotinib ( 7.3% ) or placebo ( 5.6% ), but there were more serious infections in the Decernotinib group ( 3.5% ) compared with placebo ( 1.4% ).
Through 24 weeks there were two serious adverse effects that resulted in death; one was cardiac failure in the Decernotinib 100 mg BID group ( previously reported ) and one was pancytopenia in a patient with pneumonia in the Decernotinib 200 mg QD group.
Elevations in transaminase levels and decreases in median neutrophil and lymphocyte counts were observed in the Decernotinib groups and were generally mild.
Safety profiles were comparable across groups receiving Decernotinib.
In conclusion, all tested doses of Decernotinib significantly improved signs and symptoms of rheumatoid arthritis versus placebo when administered in combination with stable background Methotrexate therapy for 24 weeks.
Decernotinib was associated with small increases in adverse reactions rates, serious infections, and mostly minor laboratory abnormalities. ( Xagena )
Decernotinib was associated with small increases in adverse reactions rates, serious infections, and mostly minor laboratory abnormalities. ( Xagena )
Source: EULAR Meeting - van Vollenhoven R et al, Ann Rheum Dis 2014;73(Suppl2)
see
WO 2007084557
http://www.google.com/patents/WO2007084557A2?cl=en
.............................................
WO 2013006634
http://www.google.com/patents/WO2013006634A2?cl=en

Formula I is:
The present invention provides a process for preparing (R)-2-(2-(lH-pyrrolo[2,3- b]pyridin-3-yl)pyrimidin-4-ylamino)-2-methyl-N-(2,2,2-trifluoroethyl)butanamide of Formula la:
la
comprising the steps of:
ivb) reacting lH-pyrrolo[2,3-b]pyridine (5a) with p-toluenesulfonyl chloride in the presence of an organic solvent to generate l-tosyl-lH-pyrrolo[2,3-b]pyridine (9a)
5a 9a
vb) reacting l-tosyl-lH-pyrrolo[2,3-b]pyridine (9a) in an organic solvent with N-bromosuccinimide to generate 3-bromo-l-tosyl-lH-pyrrolo[2,3-b]pyridine (7a)
vi) reacting 3-bromo-l-tosyl-lH-pyrrolo[2,3-b]pyridine (7a) with triisopropyl borate in the presence of a strong lithium base in an organic solvent to generate
l-tosyl-lH-pyrrolo[2,3-b]pyridin-3-ylboronic acid (8a) 0H
8a
vii) esterifying l-tosyl-lH-pyrrolo[2,3-b]pyridin-3-ylboronic acid (8a) with pinacolate alcohol in an organic solvent to generate
3 -(4,4,5 ,5 -tetramethyl- 1 ,3 ,2-dioxaborolan-2-yl)- 1 -tosyl- 1 H-pyrrolo[2,3 -bjpyridine (la) :
viiib) reacting 2,4-dichloropyrimidine (11a) with a hydrochloride salt of D-isovaline (15a) under coupling condition to generate a compound of Formula 2a
11a 2a
ixb) reacting the compound of Formula 2a with HC1 to generate the hydrochloride salt of the compound of Formula 2a;
i) reacting the compound of Formula la with the compound of Formula 2a with in the presence of water, an organic solvent, an inorganic base, and a transition metal catalyst to generate a compound of Formula 3a,
ii) deprotecting the compound of Formula 3a under basic conditions to generate a compound of Formula 4a
4a ; and iii) reacting the compound of Formula 4a with 2,2,2-trifluoroethylamine in the presence of a coupling agent and an organic solvent to generate the compound of Formula la.
![Figure imgf000093_0002]()
- l13C4, 15N2]
![Figure imgf000095_0001]()
la
comprising the steps of:
ivb) reacting lH-pyrrolo[2,3-b]pyridine (5a) with p-toluenesulfonyl chloride in the presence of an organic solvent to generate l-tosyl-lH-pyrrolo[2,3-b]pyridine (9a)
5a 9a
vb) reacting l-tosyl-lH-pyrrolo[2,3-b]pyridine (9a) in an organic solvent with N-bromosuccinimide to generate 3-bromo-l-tosyl-lH-pyrrolo[2,3-b]pyridine (7a)
vi) reacting 3-bromo-l-tosyl-lH-pyrrolo[2,3-b]pyridine (7a) with triisopropyl borate in the presence of a strong lithium base in an organic solvent to generate
l-tosyl-lH-pyrrolo[2,3-b]pyridin-3-ylboronic acid (8a) 0H
8a
vii) esterifying l-tosyl-lH-pyrrolo[2,3-b]pyridin-3-ylboronic acid (8a) with pinacolate alcohol in an organic solvent to generate
3 -(4,4,5 ,5 -tetramethyl- 1 ,3 ,2-dioxaborolan-2-yl)- 1 -tosyl- 1 H-pyrrolo[2,3 -bjpyridine (la) :
viiib) reacting 2,4-dichloropyrimidine (11a) with a hydrochloride salt of D-isovaline (15a) under coupling condition to generate a compound of Formula 2a
11a 2a
ixb) reacting the compound of Formula 2a with HC1 to generate the hydrochloride salt of the compound of Formula 2a;
i) reacting the compound of Formula la with the compound of Formula 2a with in the presence of water, an organic solvent, an inorganic base, and a transition metal catalyst to generate a compound of Formula 3a,
ii) deprotecting the compound of Formula 3a under basic conditions to generate a compound of Formula 4a
4a ; and iii) reacting the compound of Formula 4a with 2,2,2-trifluoroethylamine in the presence of a coupling agent and an organic solvent to generate the compound of Formula la.

- l13C4, 15N2]

.........................................................................
WO 2013070606
http://www.google.com/patents/WO2013070606A1?cl=en
..........................................................
patent WO2014074471
WO2014074471 claiming use of heterocyclic compound (preferably decernotinib) for treating psoriasis. Vertex is developing decernotinib, an oral JAK 3 inhibitor, for the treatment of autoimmune and inflammatory diseases, including rheumatoid arthritis. As of July 2014, the drug is Phase 3 trials.
http://www.google.com/patents/WO2014074471A1?cl=en
Table 1:
COMPD 1 IS DECERNOTINIB
Example 1: Analytical Methods Used
[0260] (A) HPLC on C18 column. Mobile phase was acetonitrile/water/TFA (60:40:0.1). Flow rate was 1.0 mL/min. Detection at wavelength of 230 nm. Run time was 25-26 minutes.
[0261] (B) HPLC on C18 column. Mobile phase was acetonitrile/water/TFA (90: 10:0.1). Flow rate was 1.0 mL/min. Detection at wavelength of 230 nm.
[0262] (C) HPLC on a Waters XBridge Phenyl column, 4.6 x 150 mm, 3.5 μπι. Mobile phase A was water/1 M ammonium formate, pH 4.0 (99: 1). Mobile phase B was
acetonitrile/water/ 1M ammonium formate, pH 4.0 (90:9:1). Gradient 5 % to 90 % B in 15 minutes. Total run time 22 minutes. Flow rate 1.5 mL/min. Detection at UV, 245 nm.
T = 25 °C.
[0263] (D) HPLC on a Waters XBridge Phenyl column, 4.6 x 150 mm, 3.5 μπι. Mobile phase A was water/1 M ammonium formate, pH 4.0 (99: 1). Mobile phase B was
acetonitrile/water/ 1M ammonium formate, pH 4.0 (90:9: 1). Gradient 15% to 90 % B in 15 minutes. Total run time 22 minutes. Flow rate 1.5 mL/min. Detection at UV, 220 nm.
T = 35 °C.
[0264] Example 2: Preparation of Compounds of Formula I [0265] General Synthetic Scheme
[0266] The Boc-protected amino acid starting material (1) undergoes amidation in the presence of an activating agent, a coupling reagent, and the acid salt of the amine HNR7R17 to generate the Boc-protected amide intermediate (2). The amide intermediate (2) is
deprotected under acidic conditions and reacted with the halogenated heteroaryl (3) to generate the aminoheteroaryl intermediate (4). Boronated azaindole (5) is coupled with the aminoheteroaryl intermediate (4) under cross-coupling condition to generate the compound of Formula I.
.....................................................................................
Patent
http://www.google.com/patents/US8163917
| 346 | M+H393.20 | RT 1.60 | (DMSO-d6, 300 MHz) 11.95 (bs, 1H), 8.7 (d, |
| 1H), 8.25 (m, 2H), 8.12 (d, 1H), 8.02 (d, 1H), | |||
| 7.28 (s, 1H), 7.13 (dd, 1H), 6.38 (bd, 1H), 3.75 | |||
| (m, 2H), 2.06 (m, 1H), 1.83 (m, 1H), 1.46 (s, | |||
| 3H), 0.8 (t, 3H); |
| 346 |
Example 1 Preparation of Compounds of the Invention
General Synthetic Scheme
Step 1
To a stirred solution of Boc-valine (1; R1 is Me; 3.8 g, 0.02 mol), EDC (4.63 g, 0.024 mol), HOBt (4.0 g, 0.026 mol), DIEA (10.5 mL, 0.06 mol) in 100 mL of DCM is added trifluoroethylamine HCl (2.92 g, 0.022 mol). The reaction mixture is stirred for 16 h. It is concentrated to dryness and redissolved in EtOAc, washed successively with 0.5N HCl, saturated aqueous solution of NaHCO3 and brine. The organic layer is dried (Na2SO4) and concentrated in vacuo to give 5.4 g (98%) of 2 as a white solid.
Step 2
Compound 2 (5.32 g, 0.0197 mol) is deprotected with a 1:1 mixture of DCM/TFA at rt for 45 min. Concentration to dryness gives the intermediate amine that is used directly for the next step. A mixture of 5-fluoro-2,4-dichloropyrimidine (3; R is F; 3.28 g, 0.0197 mol), the crude amine TFA salt (5.25 g, 0.0197 mol) and DIEA (10.27 mL, 0.059 mol) are stirred in isopropanol at rt for 16 h. The reaction mixture is concentrated in vacuo and redissolved in EtOAc, washed successively with 0.5N HCl, saturated aqueous solution of NaHCO3 and brine. The organic layer is dried (Na2SO4) and concentrated in vacuo to give a crude oil that is subjected to chromatography (50% EtOAc/50% hexanes) to yield the desired compound 4.
Step 3
A mixture of 5 (30 mg, 0.075 mmol; prepared according to WO 2005/095400), 4 (23 mg, 0.075 mmol), Pd (Ph3P)4 (9 mg, 0.0078 mmol) and sodium carbonate 2M (115 uL, 0.23 mmol) in 1 mL of DME is microwaved at 150° C. for 10 minutes. The reaction mixture is filtered through a short pad of silica gel with 30% EtOAc-70% hexanes as eluent to provide, after concentration to dryness, the crude intermediate that is used directly for the next step.
The crude intermediate is dissolved in 1 mL of dry methanol and 200 uL of sodium methoxide in methanol 25% was added. The reaction mixture is stirred at 60° C. for 1 h and quenched with 6N HCl (154 uL). The mixture is dried under a flow of nitrogen and purified by reverse phase HPLC (10-60 MeCN/water w/0.5% TFA) to provide the desired material of formula 6a.
Compounds of formulae 6b and 6c may be prepared in an analogous manner using the appropriate starting reagents. For instance, a compound of formula 6b may generally be made by substituting Cert-butyl 2-(2,2,2-trifluoroethylcarbamoyl)pyrrolidine-1-carboxylate for compound 1, while a compound of formula 6c may generally be made by substituting tert-butyl 2-(2,2,2-trifluoroethylcarbamoyl)propan-2-ylcarbamate for compound 1.
Example 2 Analytical Results
Tables 4, 5 and 6 below depicts exemplary 1H-NMR data (NMR) and liquid chromatographic mass spectral data, reported as mass plus proton (M+H), as determined by electrospray, and retention time (RT) for certain compounds of the present invention, wherein compound numbers in Tables 4, 5 and 6 correspond to the compounds depicted in Tables 1, 2 and 3, respectively (empty cells indicate that the test was not performed):
PATENTS
4-25-2012 | Azaindoles Useful as Inhibitors of Janus Kinases | |
8-4-2010 | Azaindoles useful as inhibitors of janus kinases |
new patent
WO-2014110259
| US8450489 * | Mar 1, 2012 | May 28, 2013 | Vertex Pharmaceuticals Incorporated | Azaindoles useful as inhibitors of janus kinases |
| US8530489 * | May 22, 2012 | Sep 10, 2013 | Vertex Pharmaceuticals Incorporated | 5-cyano-4-(pyrrolo [2,3B] pyridine-3-yl)-pyrimidine derivatives useful as protein kinase inhibitors |
| US8686143 * | Oct 25, 2011 | Apr 1, 2014 | Vertex Pharmaceuticals Incorporated | Compounds useful as inhibitors of Janus kinases |
| US20120157429 * | Oct 25, 2011 | Jun 21, 2012 | Wannamaker Marion W | Compounds useful as inhibitors of janus kinases |
| US20120165307 * | Mar 1, 2012 | Jun 28, 2012 | Vertex Pharmaceuticals Incorporated | Azaindoles useful as inhibitors of janus kinases |
| US20120309963 * | May 22, 2012 | Dec 6, 2012 | Vertex Pharmaceuticals Incorporated | 5-cyano-4- (pyrrolo [2,3b] pyridine-3-yl) -pyrimidine derivatives useful as protein kinase inhibitors |
| US20130237516 * | Apr 25, 2013 | Sep 12, 2013 | Vertex Pharmaceuticals Incorporated | Azaindoles useful as inhibitors of janus kinases |
| WO2013173506A2 | May 15, 2013 | Nov 21, 2013 | Rigel Pharmaceuticals, Inc. | Method of treating muscular degradation |
| WO2005095400A1 | Mar 30, 2005 | Oct 13, 2005 | Vertex Pharma | Azaindoles useful as inhibitors of jak and other protein kinases |
| WO2007084557A2 | Jan 17, 2007 | Jul 26, 2007 | Vertex Pharma | Azaindoles useful as inhibitors of janus kinases |
| WO2013070606A1 * | Nov 6, 2012 | May 16, 2013 | Vertex Pharmaceuticals Incorporated | Methods for treating inflammatory diseases and pharmaceutical combinations useful therefor |
↧
↧
Bimatoprost « New Drug Approvals
↧
Top 10 Home Remedies For Arthritis

What is Arthritis?
Arthritis is an autoimmune disorder that causes inflammation of the joints. Areas like the jaw, elbows, knees and hips are most vulnerable. In the early stages, you may complain of pain from time to time. But being physically active gradually becomes more challenging as arthritis progresses – and as the condition worsens some find themselves home bound. In America, Arthritis is the number 1 cause of disability.
read at
http://www.herbs-info.com/blog/top-10-home-remedies-for-arthritis/
↧
Jammed Protein Signal Forces Cancer Cells to Devour Themselves
↧























































































